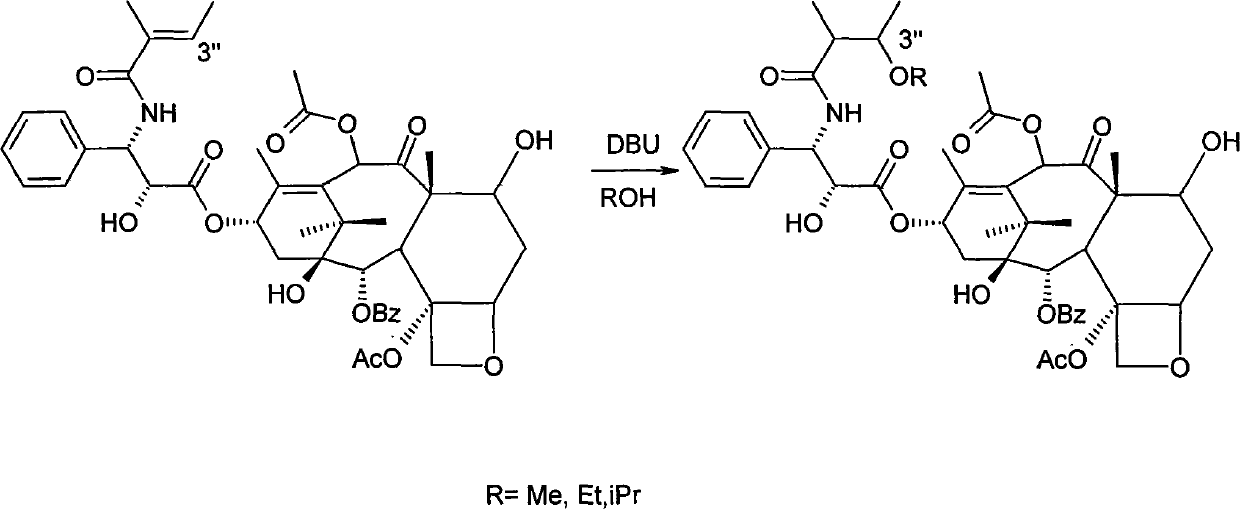
Method for separating cephalomannine from taxol
A technology of cephalomannine and alkoxy cephalomannine, which is applied in the field of chemical separation to achieve the effects of mild reaction and treatment conditions, convenient separation, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 100g of cephalomannine and paclitaxel mixture material (containing paclitaxel 9.2%, cephalomannine 61.9% (74mmol) respectively) was dissolved in 0.7L methanol, and 90ml of 1,8-diazabicyclo- Bicyclo(5,4,0)-7-undecene (containing 0.85g, 5.55mmol) in methanol. After stirring for 30 minutes, it was concentrated. After passing through a silica gel column (1.5 kg of silica gel), the gradient elution ratio of the mobile phase ethyl acetate and petroleum ether was gradually increased from 1:1 to 2:1. 37.5 g of paclitaxel was obtained, with a content of 24.1%, and a yield of 98.2%; 62.3 g of 3"-methoxycephalomannine derivatives were obtained, with a purity of 99.8%, and a yield of 98.9%.
Embodiment 2
[0023] Dissolve 100g of cephalomannine and paclitaxel mixture material (respectively containing 7.2% of paclitaxel and 77.3% of cephalomannine (92.9mmol)) in 1.4L of isopropanol, add dropwise 150mL (containing 1.18g, 11.6mmol) A solution of N-methylmorpholine in isopropanol. After stirring for 30 minutes, it was concentrated. After passing through a silica gel column (1.5 kg of silica gel), the gradient elution ratio of the mobile phase ethyl acetate and petroleum ether was gradually increased from 1:1 to 2:1. 22.6 g of paclitaxel was obtained, with a content of 31.3% (8.3 mmol), and a yield of 98.2%; 81.2 g of 3"-isopropoxy cephalomannine derivatives were obtained, with a yield of 98.0%.
Embodiment 3
[0025] Add 100 g of edible activated carbon to 2 liters of concentrated nitric acid, cool with an ice-water bath during the addition, and stir for 20 minutes. Remove the ice bath and use a sonicator instead. After ultrasonic vibration for 10 hours, filter and wash 5 liters of washing filter cake with clear water. Put the filter cake into a vacuum oven and dry it at 60° C. for 24 hours. Then, the dried activated carbon was stored in a sealed metal bottle protected by nitrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com