Diketopiperazine compound as well as composition, preparation method and application thereof
A compound and application technology, applied to diketopiperazine compounds, its preparation, its composition field, can solve the problems such as not showing antitumor activity, not yet seen etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: microbial fermentation culture and the preparation of compound I and compound II
[0066] 1. Fermentation culture and extraction of fermented products
[0067] 1) Production strain
[0068] The toxin-producing bacteria used to ferment and produce compounds I and II in this example is the Penicillium purpurogenum G591DS600S strain preserved in the General Microorganism Center of the China Microbiological Culture Collection Management Committee, and the preservation number is 4283 (CGMCC No.4283 ).
[0069] 2) Fermentation culture
[0070]According to the routine method of microbial culture, from the fungus PDA solid medium (composition: 2% glucose, 2% agar, 1.5% NaCl, prepared with 20% potato boiling liquid) test tube slant surface preserved in a refrigerator at 4°C, scrape it with an inoculation loop An appropriate amount of Penicillium purpurogenum G591DS600S was taken, streaked and inoculated on the newly prepared PDA solid medium, and activated and ...
Embodiment 2
[0088] Embodiment 2: the preparation of other formula I compound
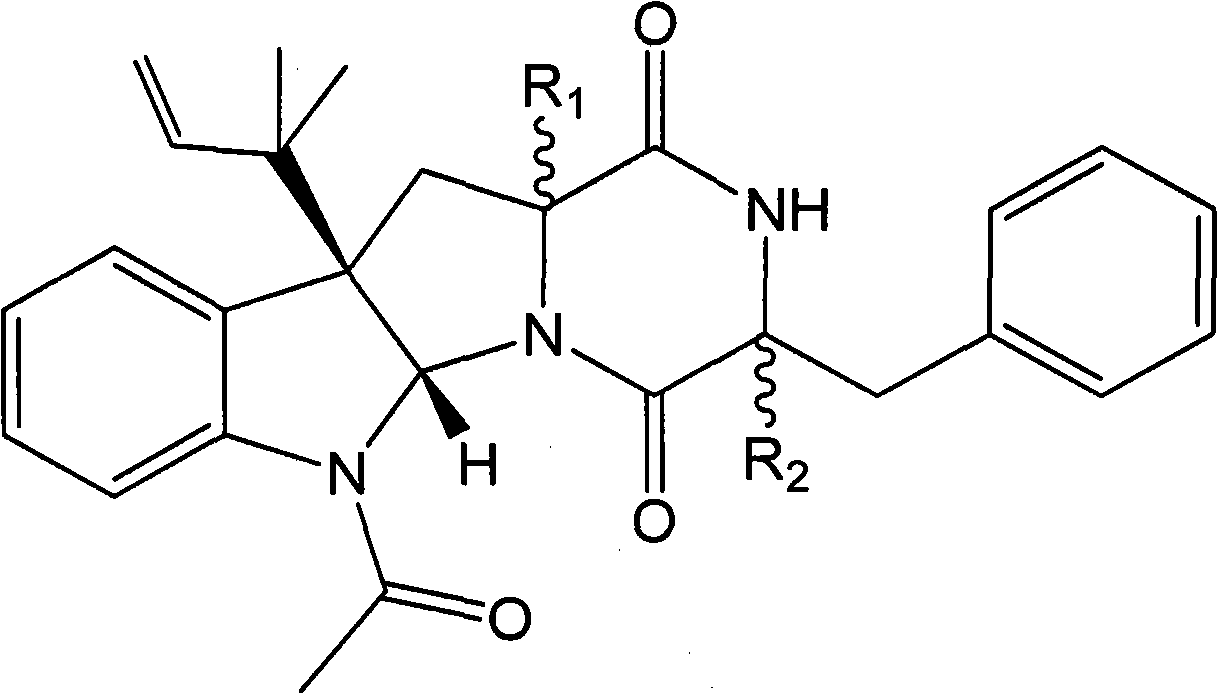
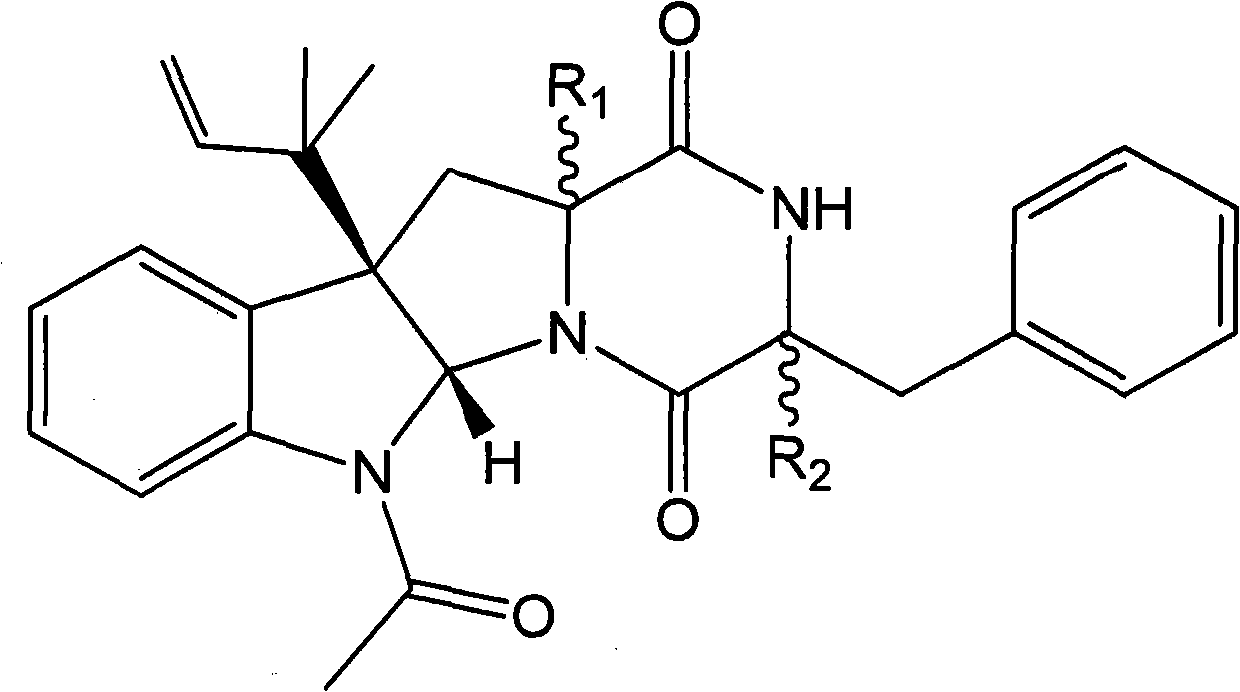
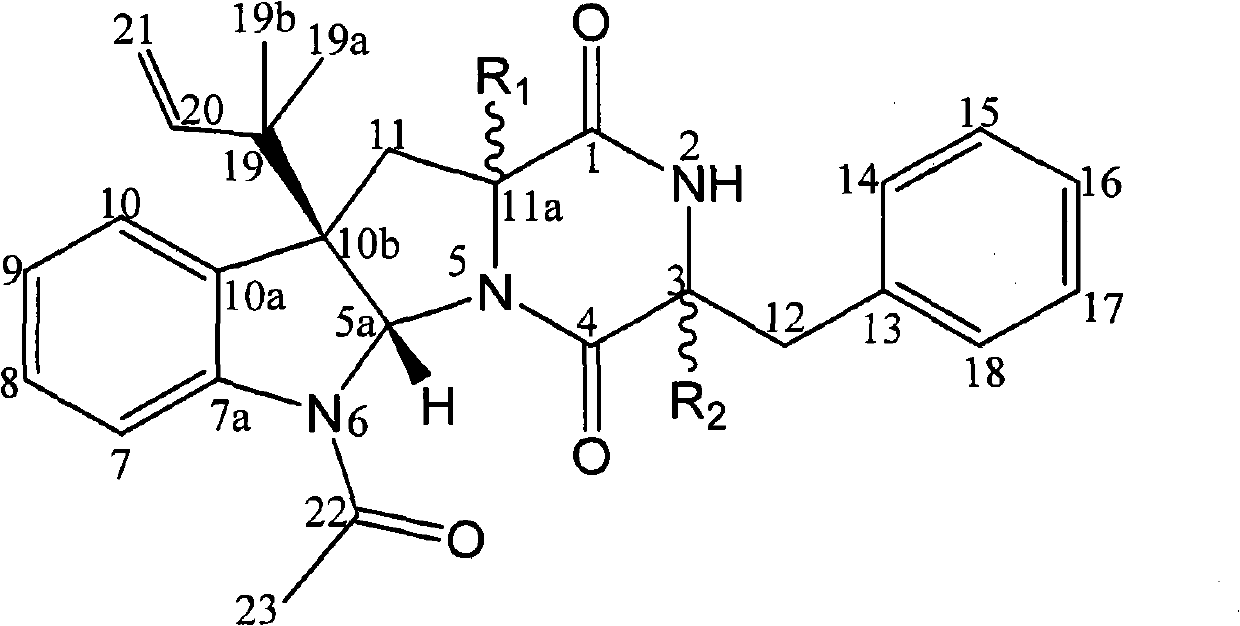
[0089] Compound I and compound II prepared in Example 1 are respectively reacted with methyl iodide (or dimethyl sulfate) under the catalysis of a suitable catalyst to obtain the methylated product of compound I (compound of formula I, wherein R 1 =β-H,R 2 =α-OCH 3 ) and the methylation product of compound II (compound of formula I, wherein R 1 =α-OCH 3 , R 2 =β-H).
[0090] Also compound I and compound II can be reacted with ethyl iodide (or diethyl sulfate) respectively to obtain the ethylated product of compound I (compound of formula I, wherein R 1 =β-H,R 2 =α-OCH 2 CH 3 ) and the ethylated product of compound II (compound of formula I, wherein R 1 =α-OCH 2 CH 3 , R 2 =β-H).
[0091] In addition, the acetylated product of compound I (compound of formula I, wherein R 1 =β-H,R 2 =α-OCOCH 3 ) and the acetylation product of compound II (compound of formula I, wherein R 1 =α-OCOCH 3 , R 2 =β...
Embodiment 3
[0094] Embodiment 3: Antitumor activity test of compound I and compound II and derivatives thereof
[0095] 1. Experimental materials
[0096] 1) Preparation of the tested sample solution
[0097] The test samples are the pure compound I and compound II separated and purified in the above example 1, the methylated product and acetylated product of the compound I prepared in the above example 2 and the methylated product and the acetylated product of the compound II . Precisely weigh an appropriate amount of sample, and use methanol to make a solution of the required concentration for testing the activity.
[0098] 2) Subculture of cell lines and cells
[0099] The human myelogenous chronic leukemia K562 cell line was used for the activity test. K562 cells were routinely passaged with RPMI-1640 medium containing 10% fetal bovine serum and 100 μg / ml penicillin and streptomycin, and maintained at 37°C in a cell culture incubator with 5% carbon dioxide.
[0100] 2. Activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com