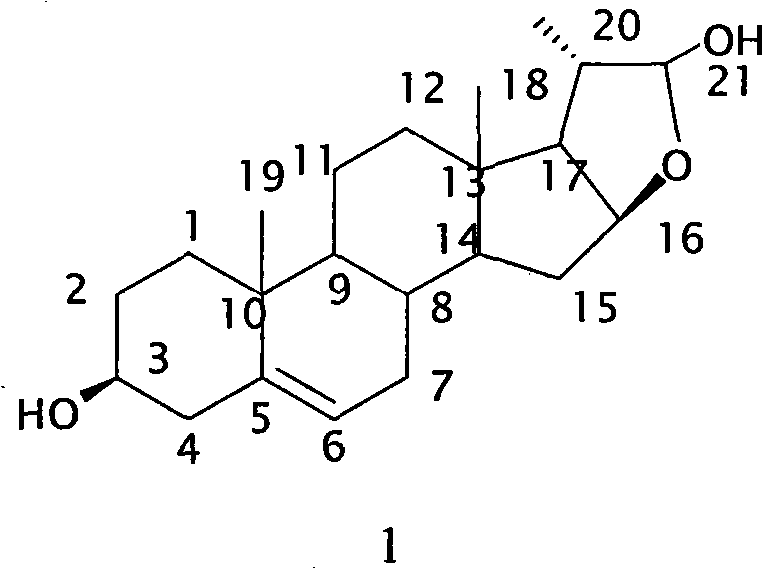
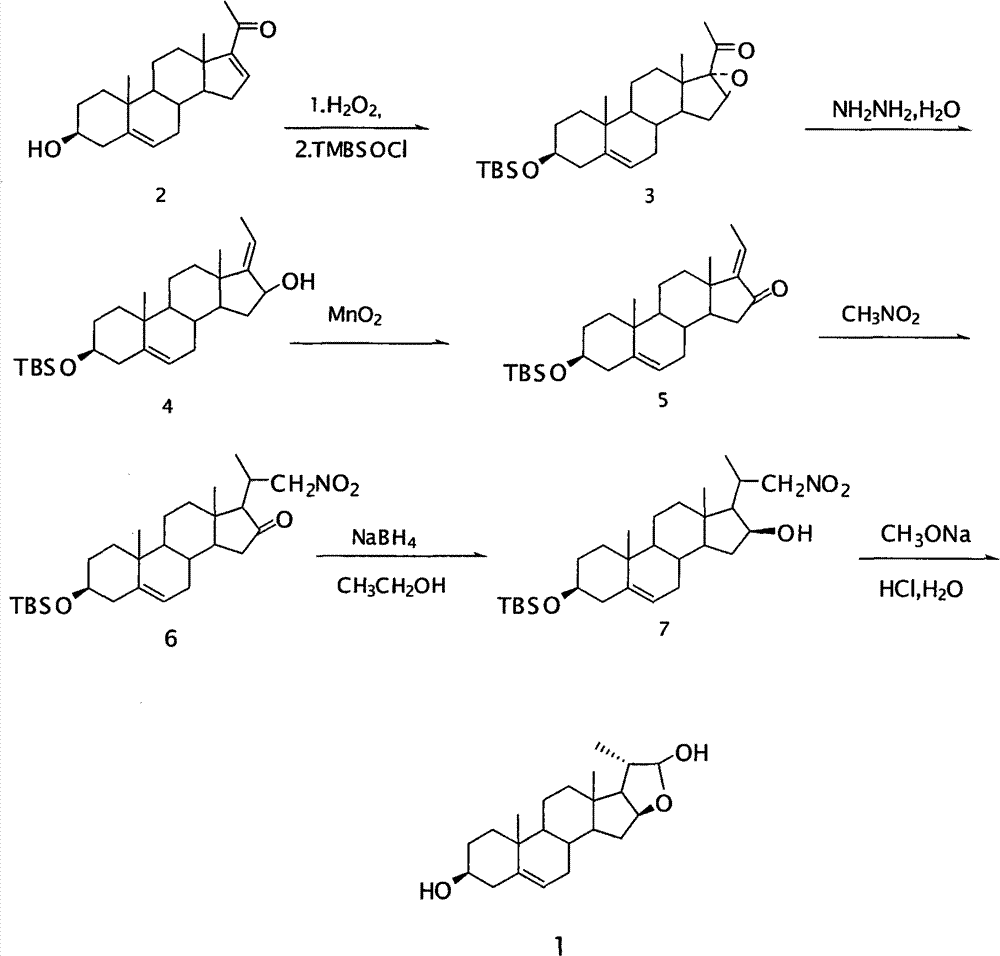
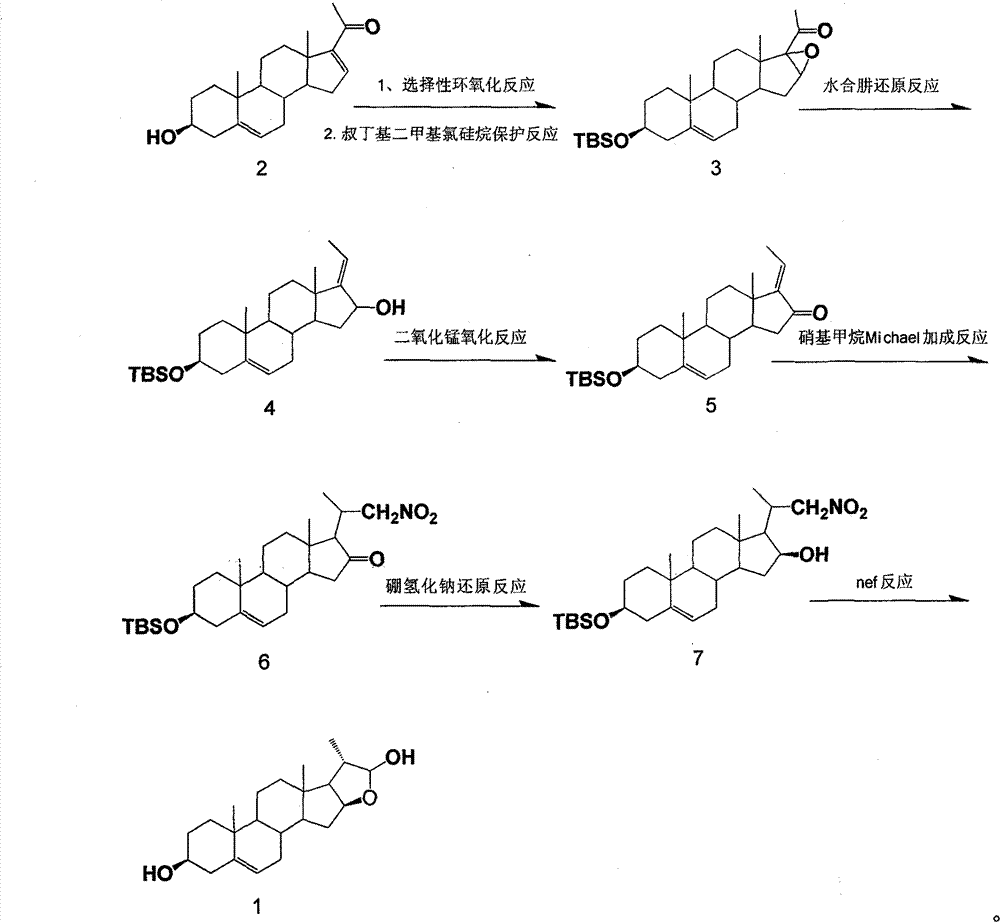
Method for synthesizing 16beta,21-epoxy-20S-methyl-alkene-3beta,21beta-pregnane-diol
An epoxidation reaction and compound technology, applied in the direction of steroids, organic chemistry, etc., to achieve the effect of good stereochemical selectivity, convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] 3β-tert-butyldimethylsilyloxy-16α, 17α-epoxy-5-ene-20-progesterone (Compound 3) Preparation:
[0017] In a 1000ml reaction flask, add raw compound 2, 30g (91.5mmol) and 600ml of methanol, stir, and at 0°C to 5°C, add an aqueous solution of NaOH (9.6g of NaOH dissolved in 60ml of water), 120ml of H 2 o 2 , the system was cloudy, and reacted for 7 hours at 0°C to 25°C, the raw materials disappeared, and the reaction was stopped. Pour the reaction night into 900ml of water, let stand overnight, filter with suction, and dry to obtain 26.7g of crude epoxidized product with a yield of 96%. Melting point: 174.3-177.8°C.
[0018] Dissolve 26.7 g of the obtained epoxidized crude product in 500 ml of dichloromethane, add 14.75 g (97.2 mmol) of tert-butyldimethylsilyl chloride, the system is always clear, add 13.88 g (204.93 mmol) of imidazole, and it becomes turbid. After stirring at room temperature for 18 hours, the reaction was complete. Spin off the dichloromethane liquid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com