Method for preparing lithium alkoxide
A technology of lithium alkoxide and lithium tert-butoxide, which is applied in the preparation of metal alkoxides, etc., can solve the problems of reduced yield, small atomic weight of metal lithium, and hidden dangers to the life safety of environmental operators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
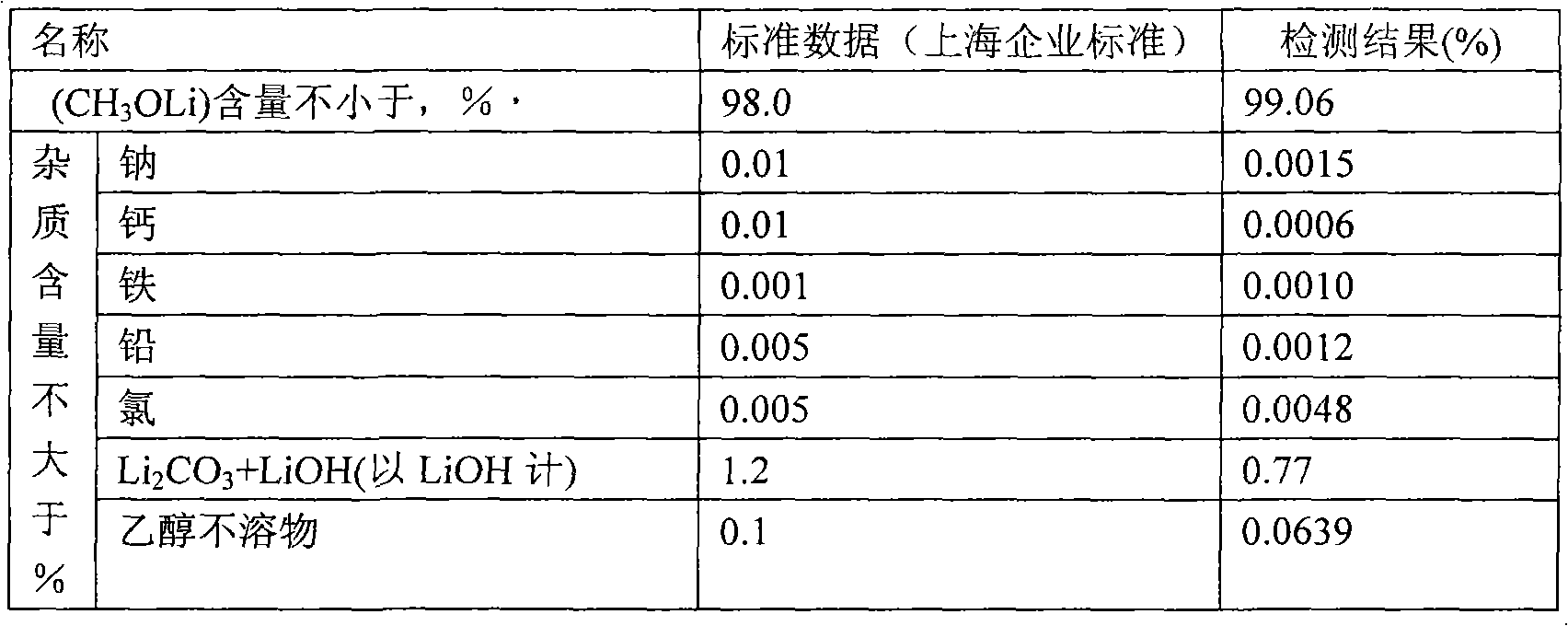
[0020] Weigh 2000g of tetrahydrofuran into a 20L titanium bucket, and gradually add 800.5g of lithium tert-butoxide under stirring until the solution is completely clear. Under stirring, a total of 352.4 g of anhydrous methanol was added every half hour for 5 times. After the anhydrous methanol was completely added, the stirring was continued for 3 hours, and the material was transferred to a centrifuge under argon protection for separation. The separated solid was transferred to a vacuum oven for 8 hours at 120°C, and the vacuum degree was -0.074MPa. After crushing, a white powdery solid was obtained, weighing 375.2g. The mass analysis was carried out using (Shanghai enterprise standard), and the results were as follows:
[0021]
Embodiment 2
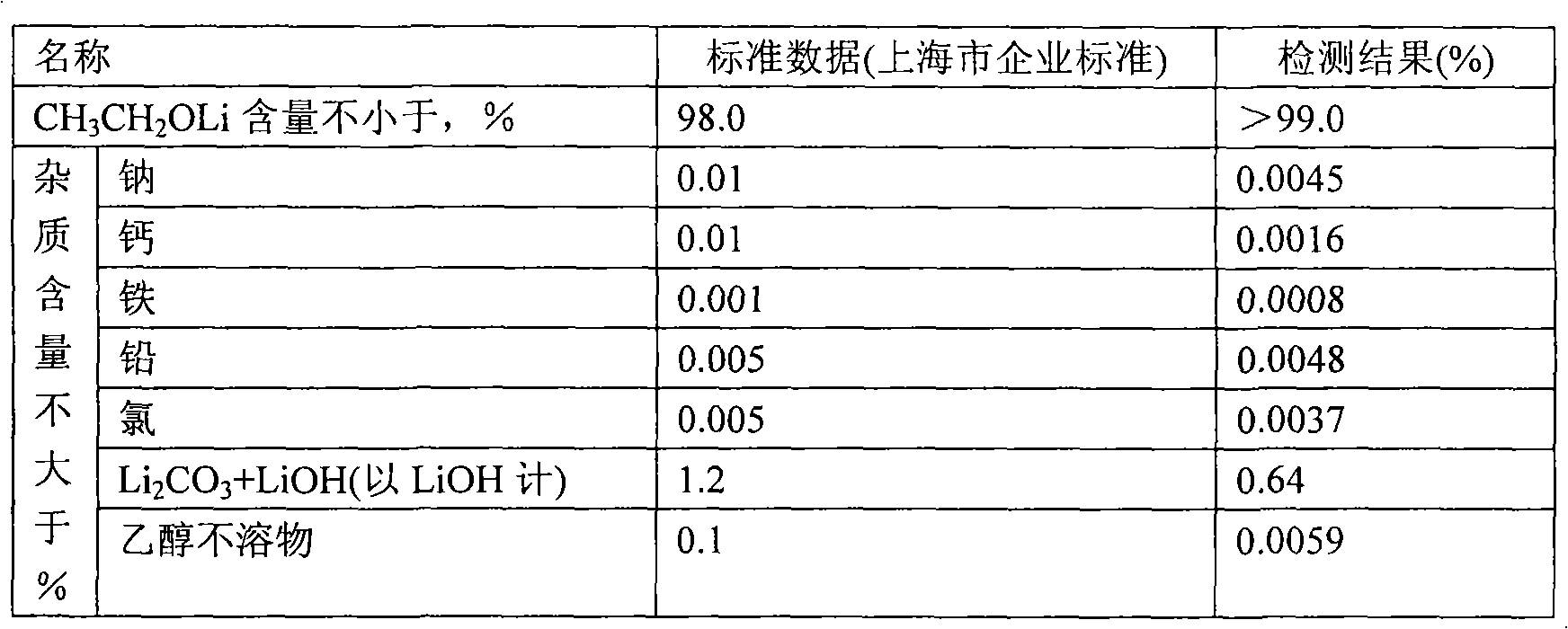
[0023] Weigh 1000g of tetrahydrofuran into a 20L titanium bucket, and gradually add 400.3g of lithium tert-butoxide under stirring until the solution is completely clear. Under stirring, a total of 253 g of absolute ethanol was added every half hour for 5 times. After complete addition of absolute ethanol, the stirring was continued for 2 hours, and then the material was transferred to a centrifuge under the protection of argon for separation. The separated solid was transferred to a vacuum oven at 120°C for 6 hours and the vacuum degree was -0.074MPa. After crushing, a light yellow powdery solid was obtained, weighing 255.5g. The mass analysis results were as follows:
[0024]
Embodiment 3
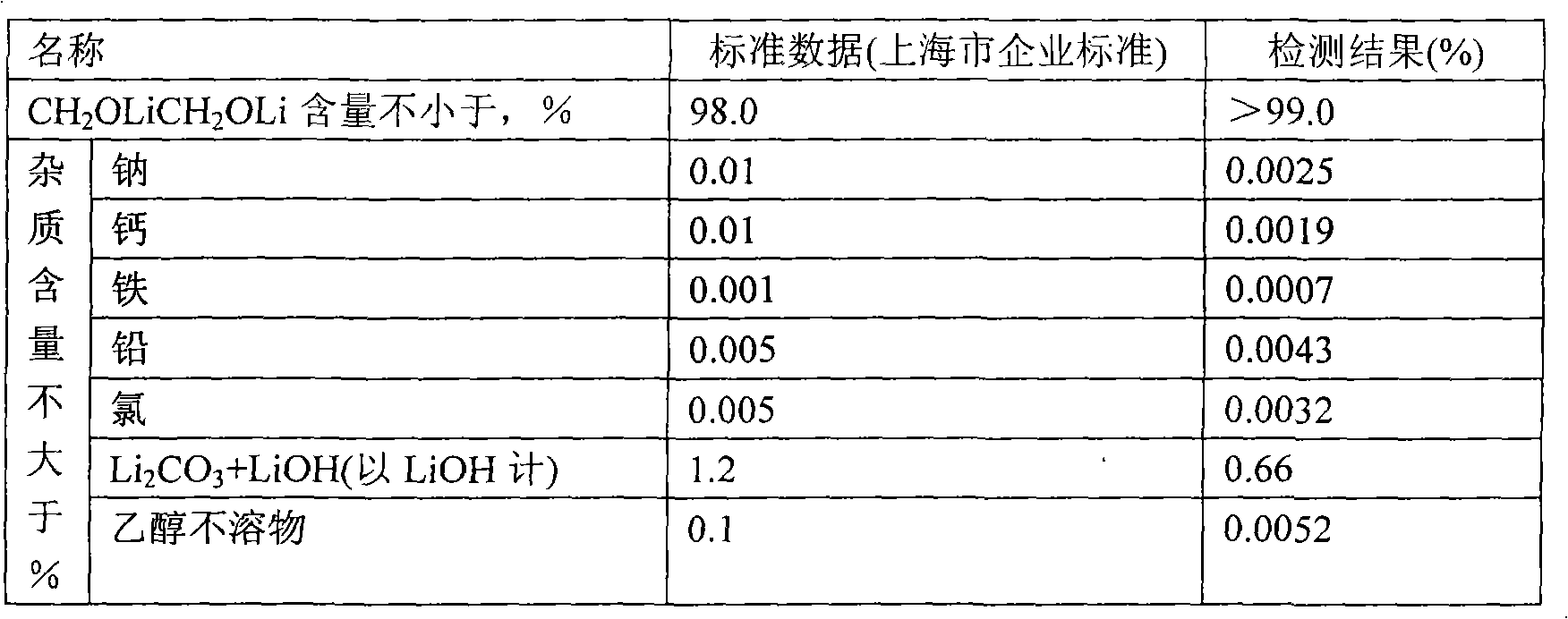
[0026] Weigh 2000g of tetrahydrofuran into a 20L titanium bucket, and gradually add 300.5g of lithium isopropoxide under stirring until the solution is completely clear. Under stirring, a total of 253 g of anhydrous ethylene glycol was added every half hour in 5 times. After the anhydrous ethylene glycol was completely added, the stirring was continued for 3 hours, and the material was transferred to a centrifuge under the protection of argon for separation. The separated solid was transferred to a vacuum oven at 150°C and dried for 12 hours with a vacuum degree of -0.074MPa. After crushing, a light yellow powdery solid was obtained, weighing 184.4g. The mass analysis results were as follows:
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com