Metal coordination polymer and preparation method and application thereof
A metal coordination and polymer technology, applied in the direction of copper organic compounds, nickel organic compounds, etc., can solve the problems of low electrical conductivity and low seebeck coefficient, and achieve the effects of stable properties, excellent thermoelectric performance, and low thermal conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The method for preparing the metal coordination polymer based on ethylene tetrathiolate provided by the invention is to use 1,3,4,6-tetrathiadicyclopentene-2,5-dione as raw material, through alkali and Metal salts generate metal complexes, and then generate charge transfer complexes through ammonium salts or other metal ions, and oxidize them to obtain the final product. The synthesis route is as follows:
[0034]
[0035] The material prepared by the present invention can be tested for thermoelectric performance by the following method: testing the thermoelectric performance of the product. The sample is pressed into tablets to obtain a regular cuboid, which is measured with a four-electrode method, and the resistance at different temperatures is obtained by measuring with a low-temperature electrical and magnetic performance system. The sample was also pressed into tablets, and the seebeck coefficient was measured on the sample with the Seebeck Measurement System (...
Embodiment 1
[0036] Embodiment 1, the metal coordination polymer based on ethylene tetrathiolate shown in the preparation formula a
[0037]
[0038] (formula a)
[0039] Put 1,3,4,6-tetrathiadicyclopentene-2,5-dione (170mg) into a 50ml three-necked flask, add 20ml of nitrogen-protected methanol solution with a concentration of 12mg / mL sodium methoxide, room temperature Stir under nitrogen protection for 1 hour. Inject 15 ml of methanol solution of nickel chloride hexahydrate with a concentration of 13 mg / mL into the above solution, and stir at room temperature for half an hour. Add 20 mL of tetradecyltrimethylammonium bromide methanol solution with a concentration of 27 mg / mL, and stir at room temperature for half an hour. The solid was obtained by suction filtration and washed with methanol and water. Vacuum dry overnight. The obtained solid was dissolved in 10 mL of methanol at a concentration of 4 mg / mL, 20 mg of iodine was added, and oxidized at room temperature for 3 hours to ...
Embodiment 2
[0046] Embodiment 2, the metal coordination polymer based on ethylene tetrathiolate shown in synthetic formula b
[0047]
[0048] (formula b)
[0049] Put 1,3,4,6-tetrathiadicyclopentene-2,5-dione (261mg) into a 50ml three-necked flask, add 20ml of methanol solution of sodium methoxide with a concentration of 15mg / mL under nitrogen protection, Stir at room temperature for 1 hour under nitrogen protection. Inject 15 ml of a methanol solution of nickel chloride hexahydrate with a concentration of 20 mg / mL into the above solution, and stir at room temperature for 1 hour. The solid was obtained by suction filtration and washed with methanol and water. Vacuum dry overnight. The obtained solid was dissolved in 10 mL of methanol at a concentration of 6 mg / mL, and oxidized in air at room temperature for 13 days to obtain 200 mg of an ethylene tetrathiolate-based metal coordination polymer represented by formula b, with a yield of 78.0%.
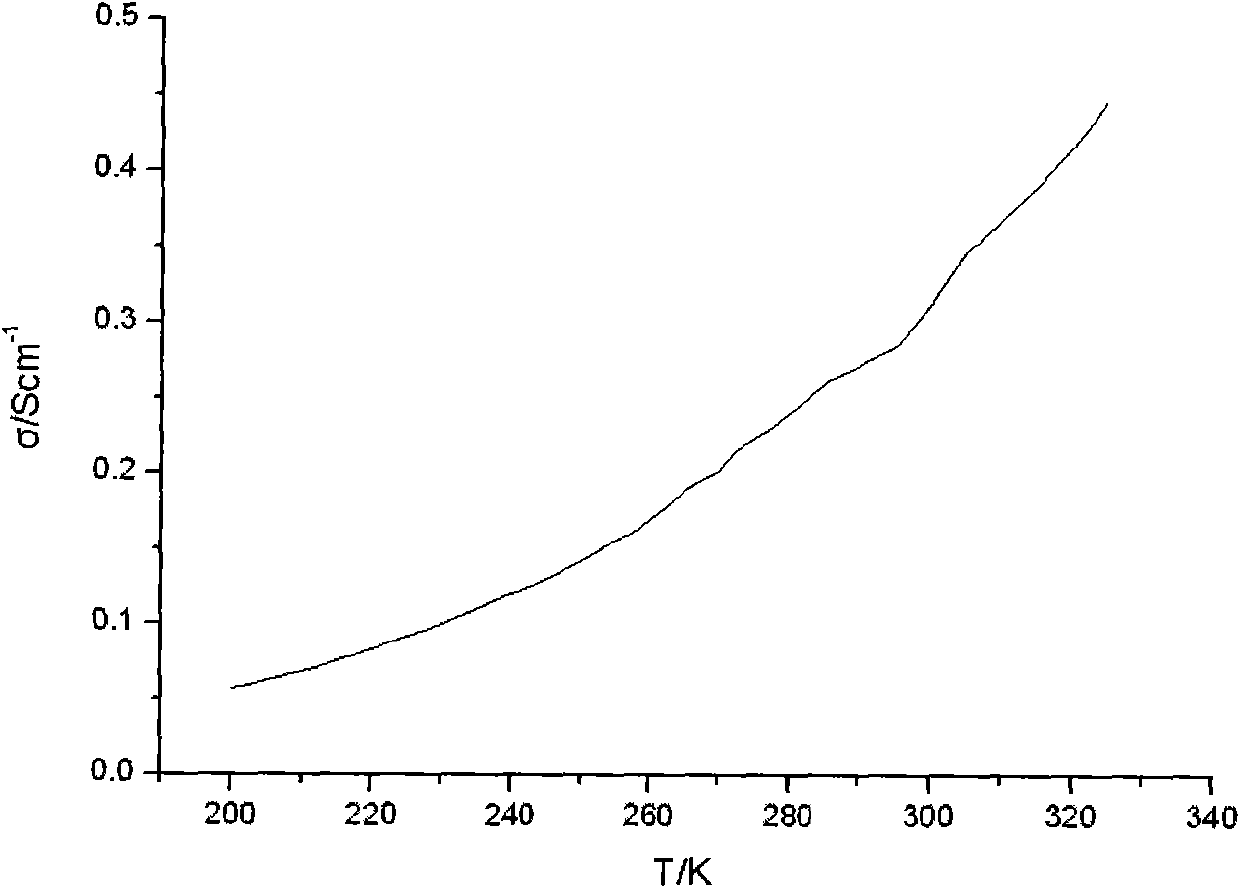
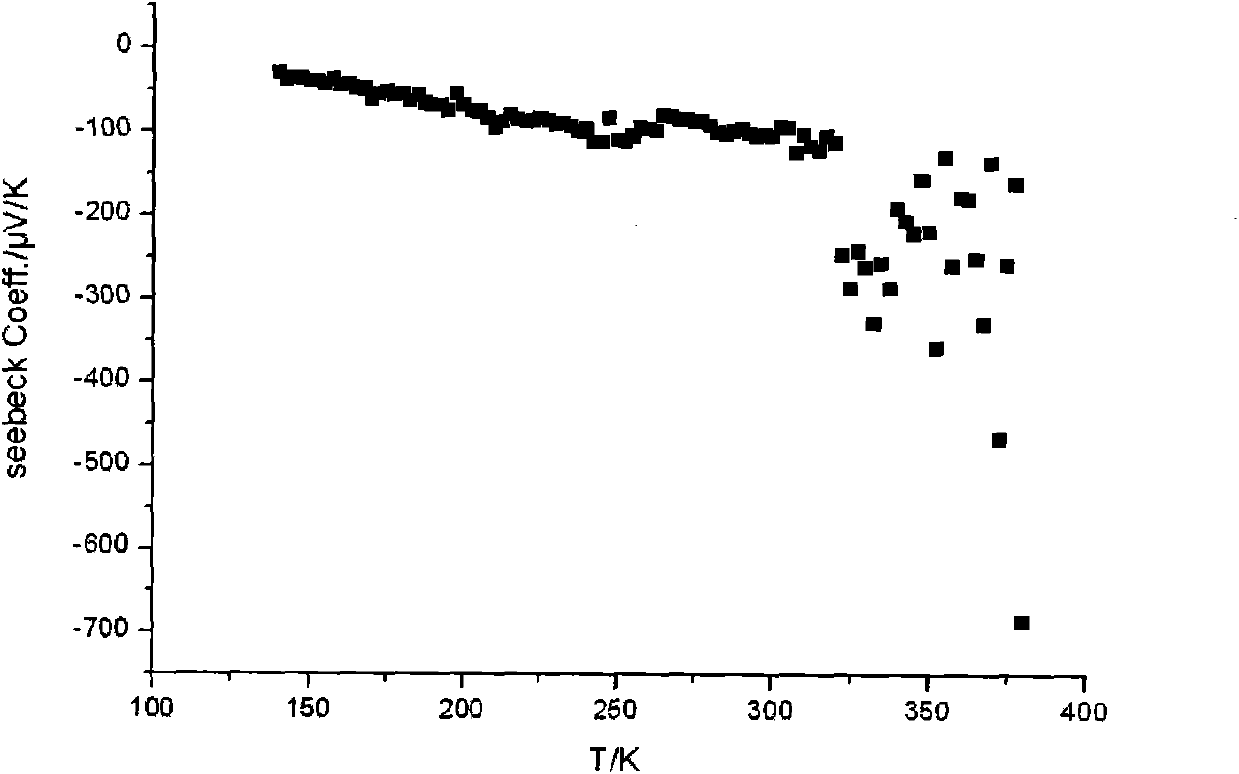
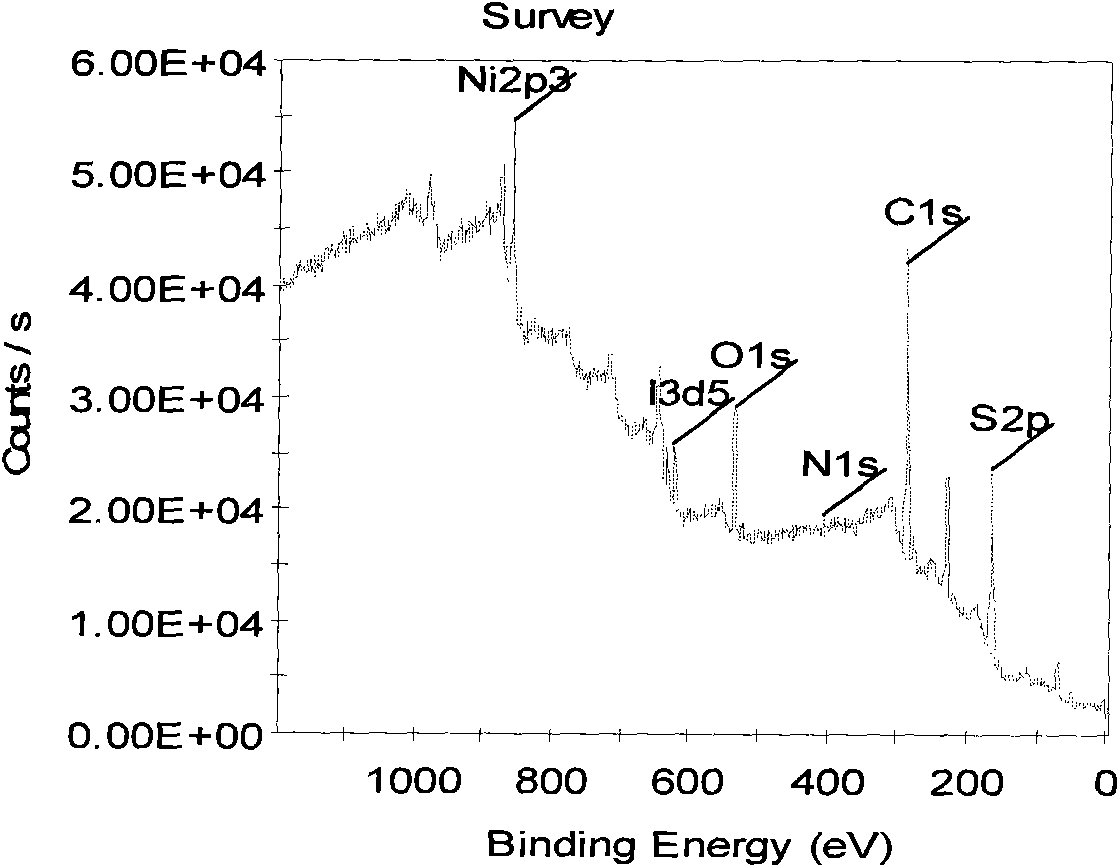
[0050] Figure 4 It is the photoelect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Seebeck coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com