Process for preparing tetrahydrate manganese chloride by leaching out rhodochrosite with waste acid
A technology of manganese chloride tetrahydrate and rhodochrosite, applied in manganese halide and other directions, can solve the problems of waste of recyclable resources, energy, unreasonable utilization of resources, etc., and achieve the effect of low production cost and good comprehensive benefit.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
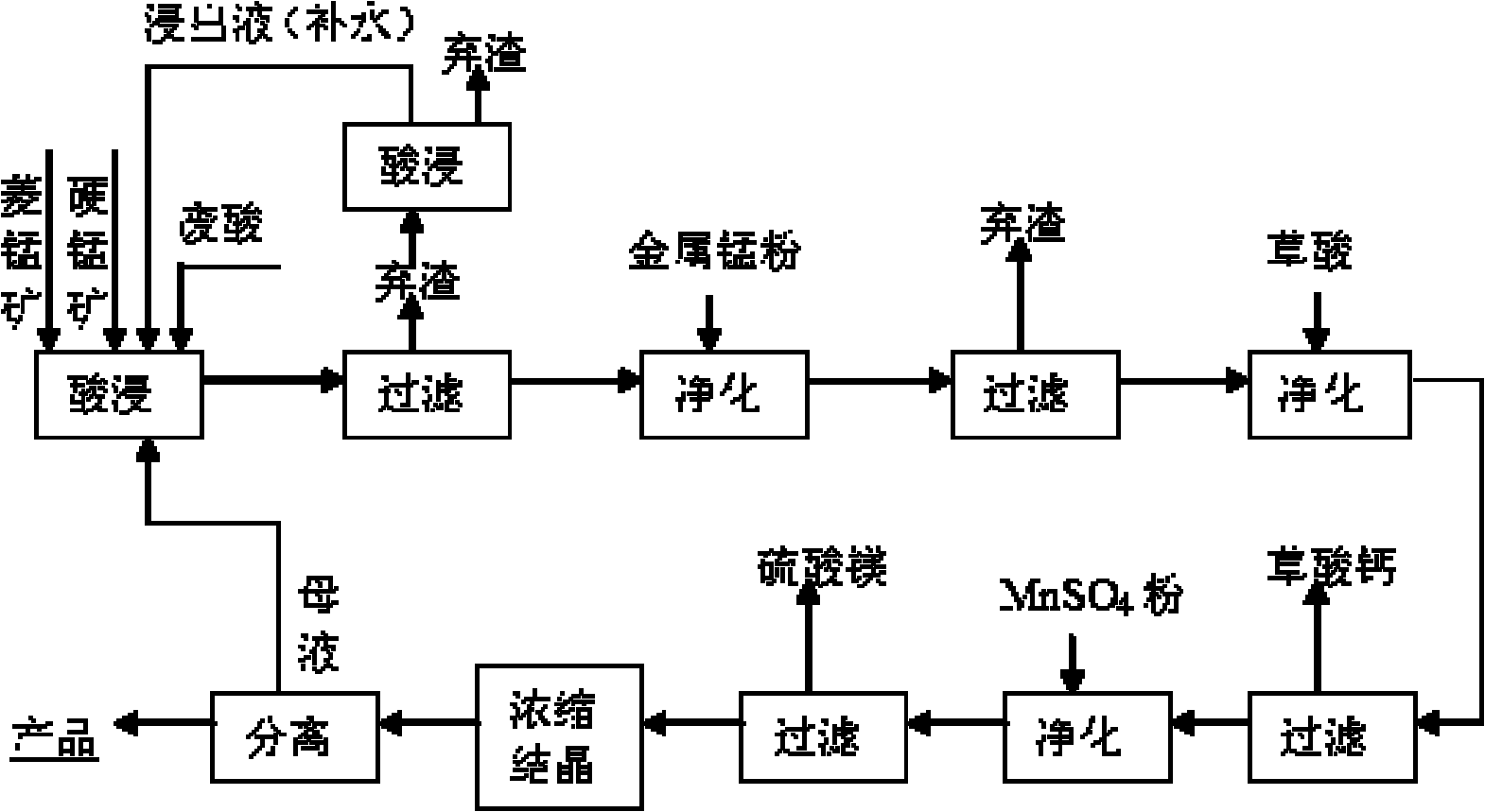
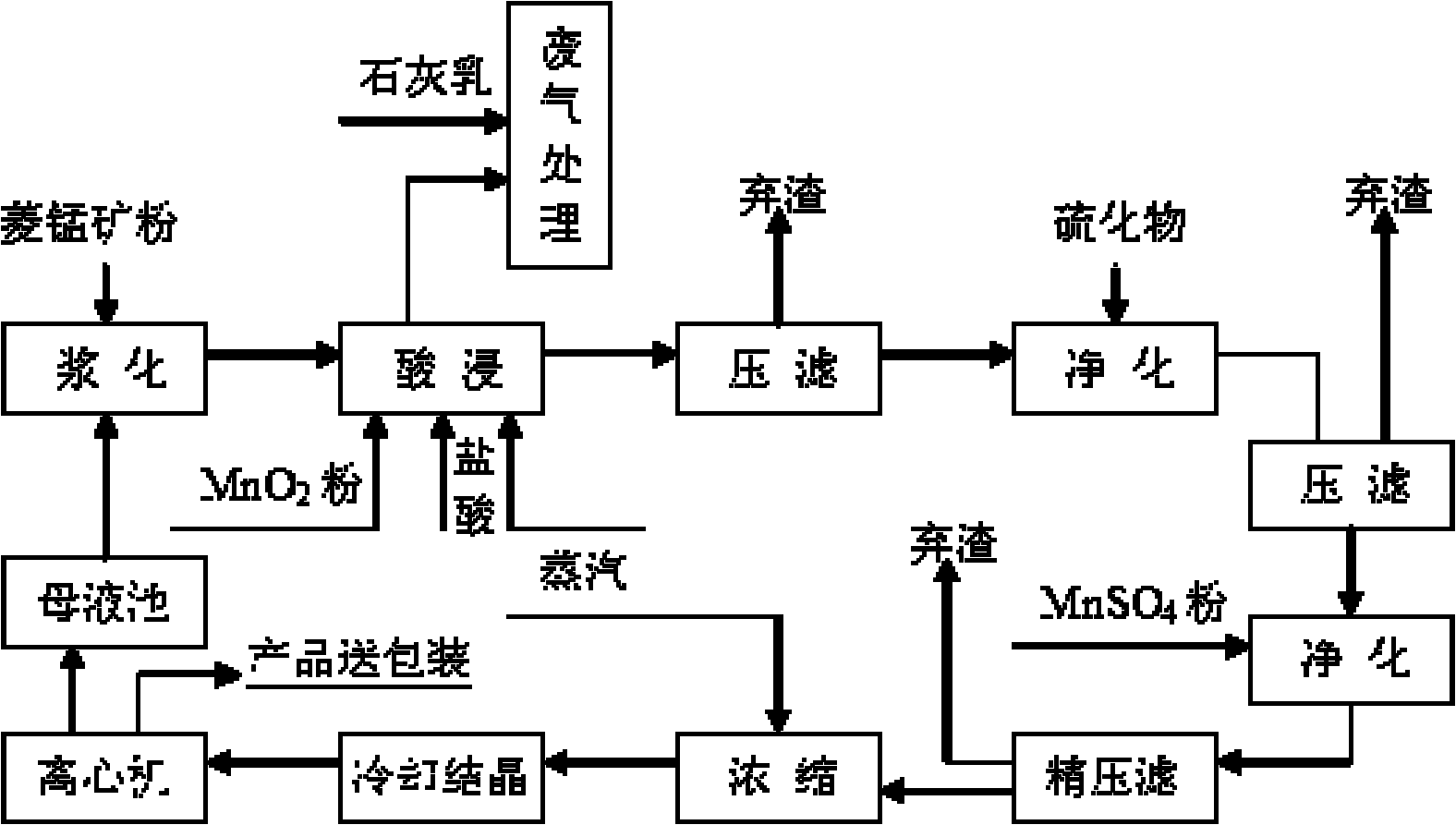
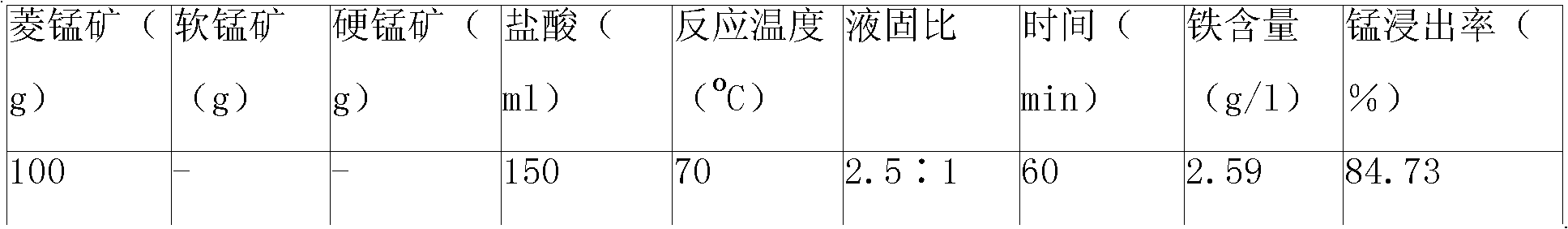
[0159] Embodiment 1 of the present invention: get rhodochrosite (90% particle diameter is within 100 orders), add step by step waste hydrochloric acid (concentration is 22%~25%) reclaiming from sponge titanium chlorination production and leaching 3 times, the hydrochloric acid The excess coefficient is 1.3, colemanite is added as an oxidant at the same time, the liquid-solid ratio of the reaction system is 2.5:1, the leaching reaction temperature is 80°C, the reaction time is 60 minutes, and the stirring intensity is 90 rpm; the pH value of the reaction process is controlled at 0.5-1.0, MnO in manganese ore can be made 2 Play its oxidation function very well; at the end of the reaction, use CaO as a pH regulator to adjust the pH value to 4.0-5.0, which can make the Fe in the leachate 3+ Hydrolyzed to achieve the purpose of iron removal. After the reaction, the mixed solution generated was filtered, and the filter residue was leached with acid again, the reaction temperature w...
Embodiment 2
[0161] Embodiment 2 of the present invention: get rhodochrosite, add waste hydrochloric acid (concentration is 22%~25%) step by step to leach twice, the excess coefficient of hydrochloric acid is 1.3, add coromansite simultaneously as oxidant, the liquid-solid ratio of reaction system is 2.5 : 1, the leaching reaction temperature is 80°C, the reaction time is 60 minutes, and the stirring intensity is 90 rpm; the pH value of the reaction process is controlled at 0.5~1.0, which can make the MnO 2 Play its oxidation function very well; at the end of the reaction, use CaO as a pH regulator to adjust the pH value to 4.0-5.0, which can make the Fe in the leachate 3+ Hydrolyzed to achieve the purpose of iron removal. After the reaction, the mixed solution generated was filtered, and the filter residue was leached with acid again, the reaction temperature was 60°C, and the reaction time was 30 minutes; the filtrate was added with metal manganese powder and stirred and heated at 80°C f...
Embodiment 3
[0162] Embodiment 3 of the present invention: get rhodochrosite, add waste hydrochloric acid (concentration is 22%~25%) leaching, the excess coefficient of hydrochloric acid is 1.3, add pyrolusite simultaneously as oxidant, the liquid-solid ratio of reaction system is 2.5: 1, leaching The reaction temperature is 80°C, and the reaction time is 60 minutes; the pH value of the reaction process is controlled at 0.5-1.0, which can make the MnO in duromanganese 2 Play its oxidation function very well; at the end of the reaction, use CaO as a pH regulator to adjust the pH value to 4.0-5.0, which can make the Fe in the leachate 3+ Hydrolyzed to achieve the purpose of iron removal. After the reaction, the mixed solution generated was filtered, and the filter residue was leached with acid again, the reaction temperature was 60°C, and the reaction time was 30 minutes; the filtrate was added with metal manganese powder and stirred and heated at 80°C for 1 hour to form a heavy metal precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com