Method for synthesizing Teprenone
A teprenone and catalyst technology, applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carbonyl compounds, etc., can solve the problems of increased impurities, high water solubility, low conversion rate, etc., and achieve inhibition of alcohol The generation of similar by-products, the improvement of product purity, and the effect of realizing industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
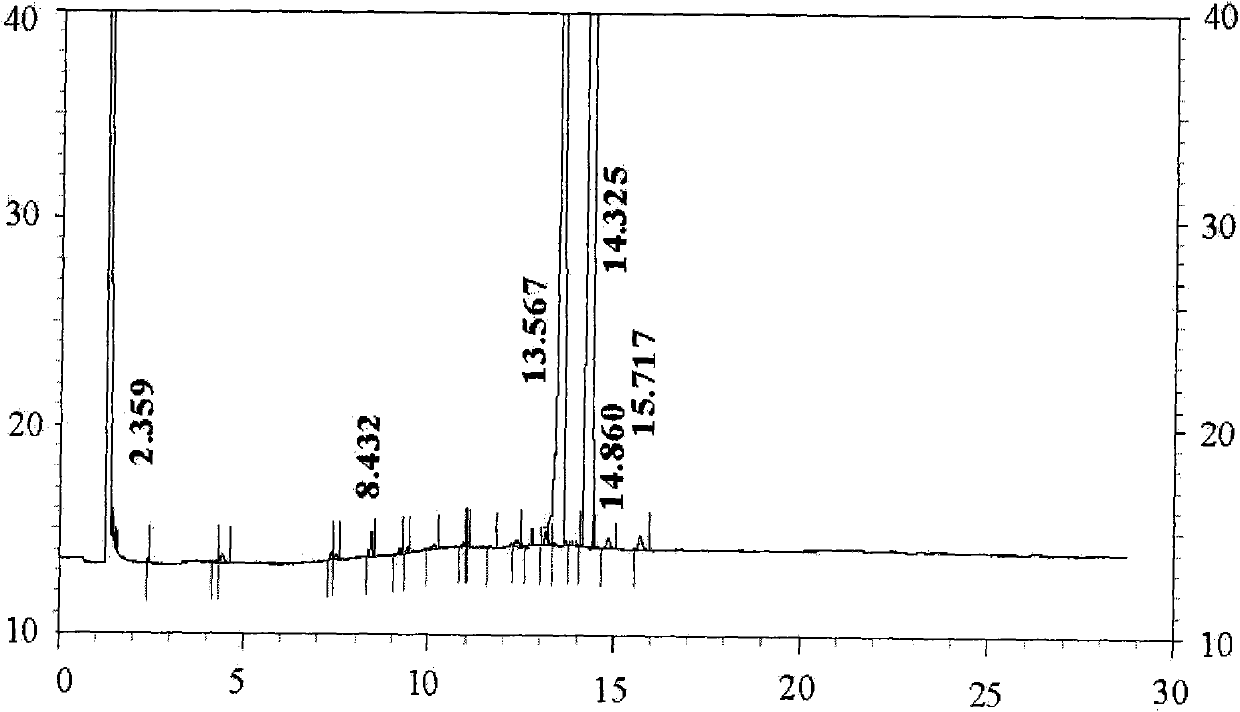
[0034] Embodiment 1 The synthesis and separation method of teprenone of the present invention
[0035] Dissolve 5.0g (24.5mmol) of aluminum isopropoxide in 20g (0.172mol) of methyl acetoacetate at 40-100°C, then distill under negative pressure at 40-120°C with a pressure of 0.03MPa, discard Slip out of the liquid to get a pretreated catalyst. Add 120g (0.413mol) of geranyl linalool, 38g (0.327mol) of methyl acetoacetate, 40g of cyclohexanone and pretreated catalyst in the three-necked flask, stir and heat up, react at 125°C for 30-60 minutes, and then increase to React at 135°C for 30-60 minutes, then react at 150°C for 2-4 hours, while the water pump 0.03MPa vacuum distills methanol and removes CO 2 , stop reaction, rapid cooling, get the crude product of teprenone, residual raw material geranyllinalool 0.85% in GC measurement reaction solution, product 95.76% (double peak), alcohols by-product accounts for 0.12% (double peak), reaction Liquid was directly added to a 1L sep...
Embodiment 2
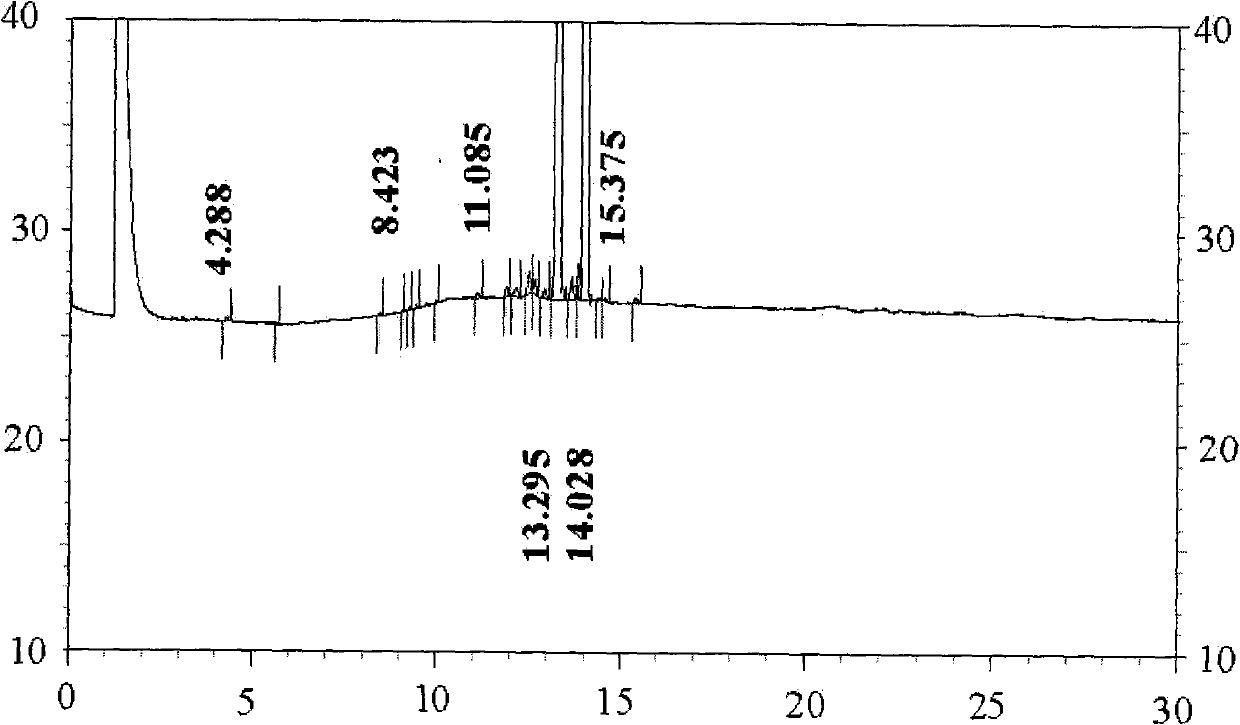
[0037] Embodiment 2 The synthesis and separation method of teprenone of the present invention
[0038]Dissolve 5.0g (24.5mmol) of aluminum isopropoxide in 38.4g (0.330mol) of methyl acetoacetate at 40-100°C, then distill under negative pressure at 40-120°C with a pressure of 0.03MPa, discard Slip out of the liquid and get the pretreated catalyst. Add 120 g (0.413 mol) of geranyl linalool, 38.4 g (0.330 mol) of methyl acetoacetate, 100 g of methyl amyl ketone and a pretreated catalyst in a three-necked flask, stir and heat up, and react at 125° C. for 30 minutes, then increase React at 135°C for 60 minutes, then react at 150°C for 3 hours, while the water pump 0.03MPa vacuum distills methanol and removes CO 2 , stop reaction, rapid cooling, get the thick product of teprenone, 0.65% of residual raw material geranyllinalool is measured in the reaction liquid by GC, product 95.9% (double peak), alcohols by-product accounts for 0.09% (double peak), reaction Liquid was directly ad...
Embodiment 3
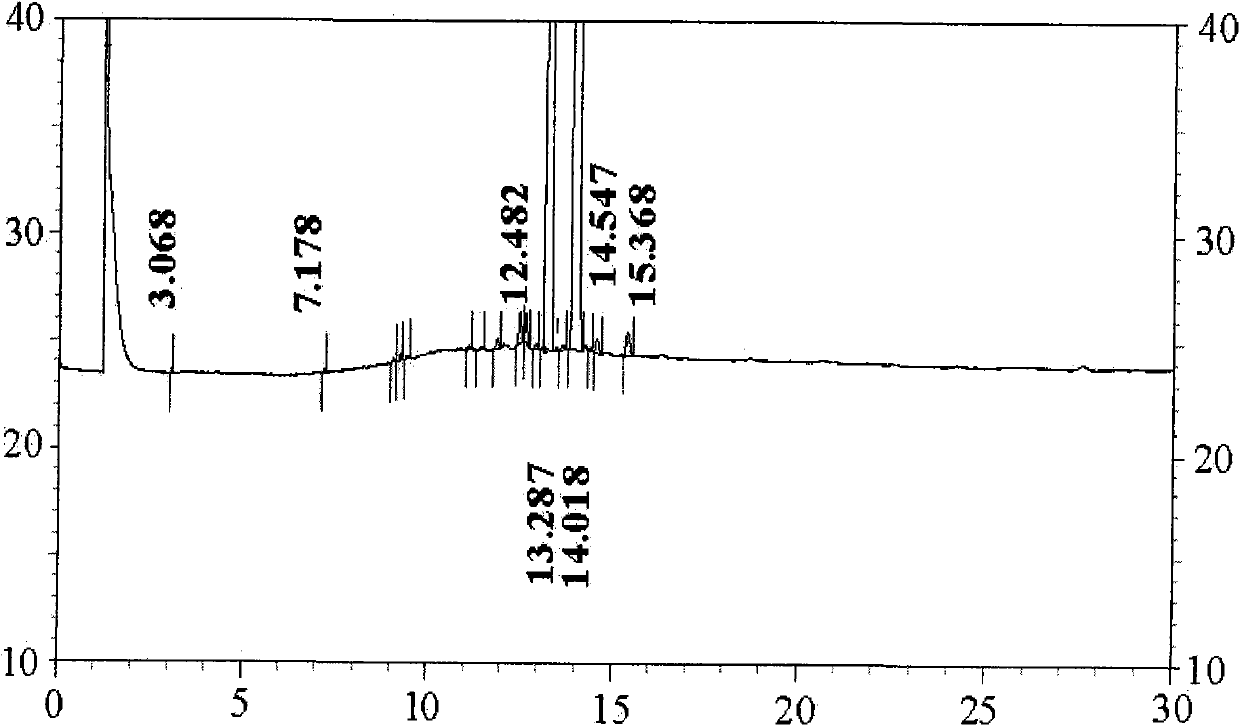
[0040] Embodiment 3 The synthesis and separation method of teprenone of the present invention
[0041] Dissolve 5.0g (24.5mmol) of aluminum isopropoxide in 19g (0.165mol) of methyl acetoacetate at 40-100°C, then distill under negative pressure at 40-120°C with a pressure of 0.03MPa, discard Slip out of the liquid and get the pretreated catalyst. Add 120g (0.413mol) of geranyl linalool, 77g (0.661mol) of methyl acetoacetate, 20g of heptanone and the pretreated catalyst in the three-necked flask, react at 125°C for 30-60 minutes, and then raise the temperature to 135°C for 30 minutes. -60 minutes, and then react at 150°C for 2-4 hours, while the water pump 0.03MPa vacuum distills methanol and removes CO 2 , stop reaction, rapid cooling, get the thick product of teprenone, residual raw material geranyllinalool 0.98% in GC measurement reaction solution, product 95.3% (double peak), alcohols by-product accounts for 0.16% (double peak), reaction Add liquid directly into a 1L separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com