Visible light free radical and cation double-effect photosensitive initiator prepared from iron arene complexes
A photocuring initiator and light source technology, which is applied in the field of new ferrocene salts, can solve the problems that visible light sources cannot be used, and achieve the effects of convenient raw material sources, easy-to-obtain raw material sources, and simple synthesis methods and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
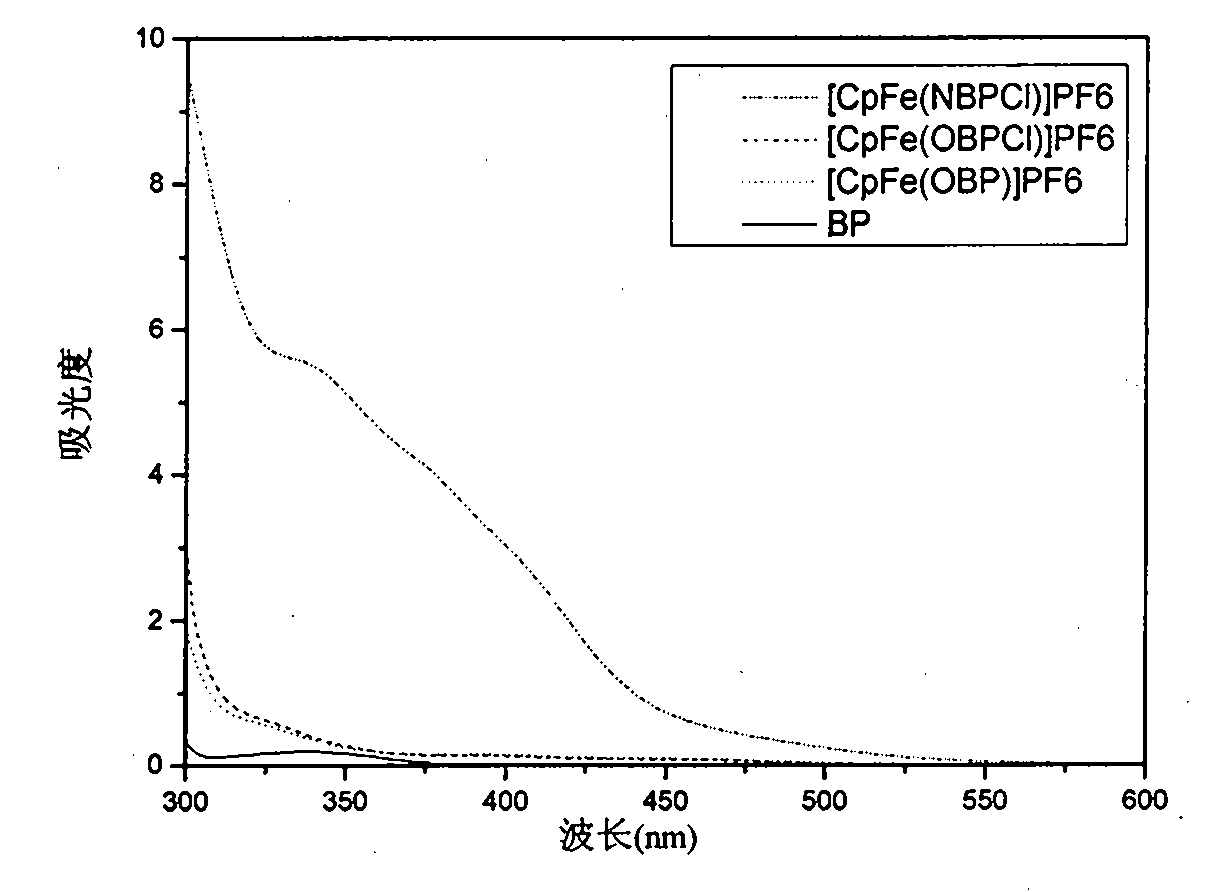
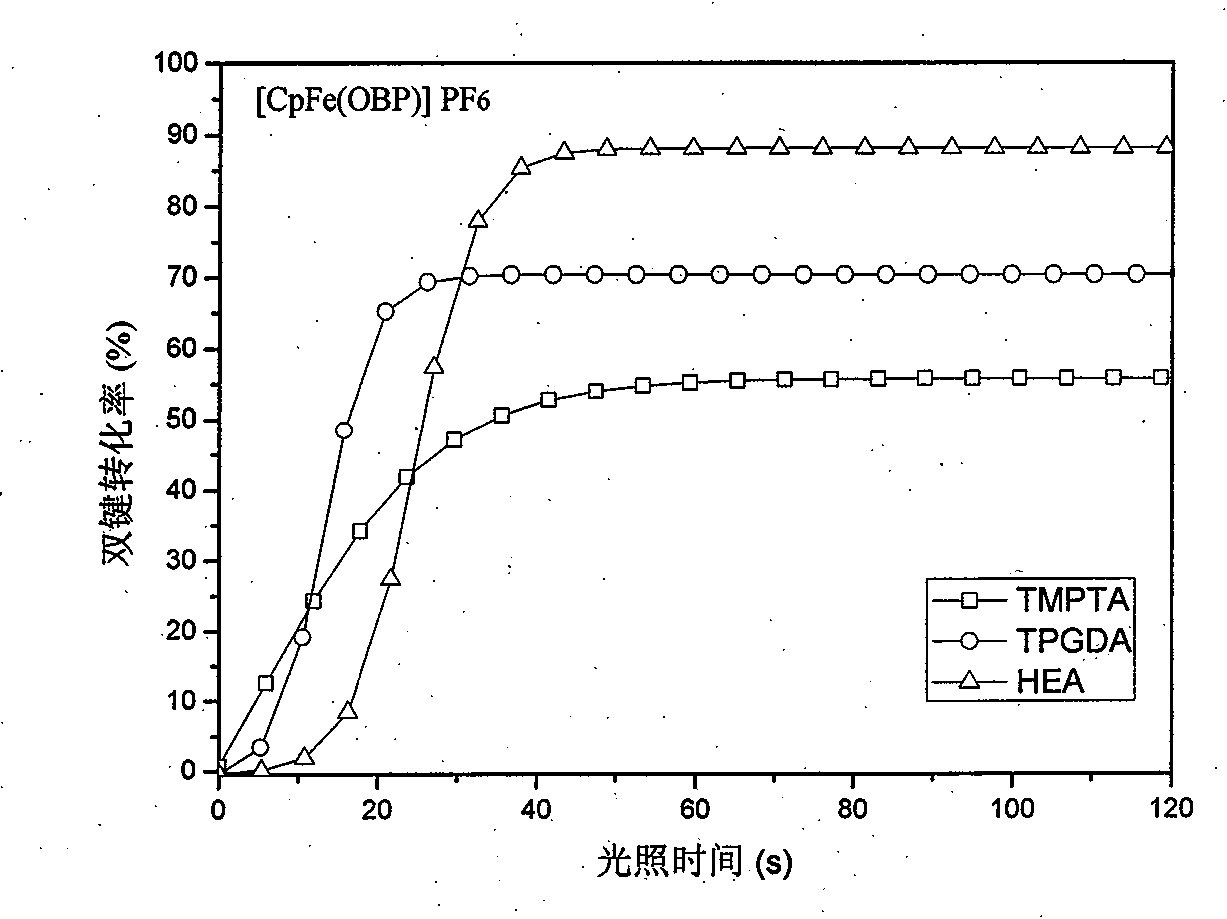
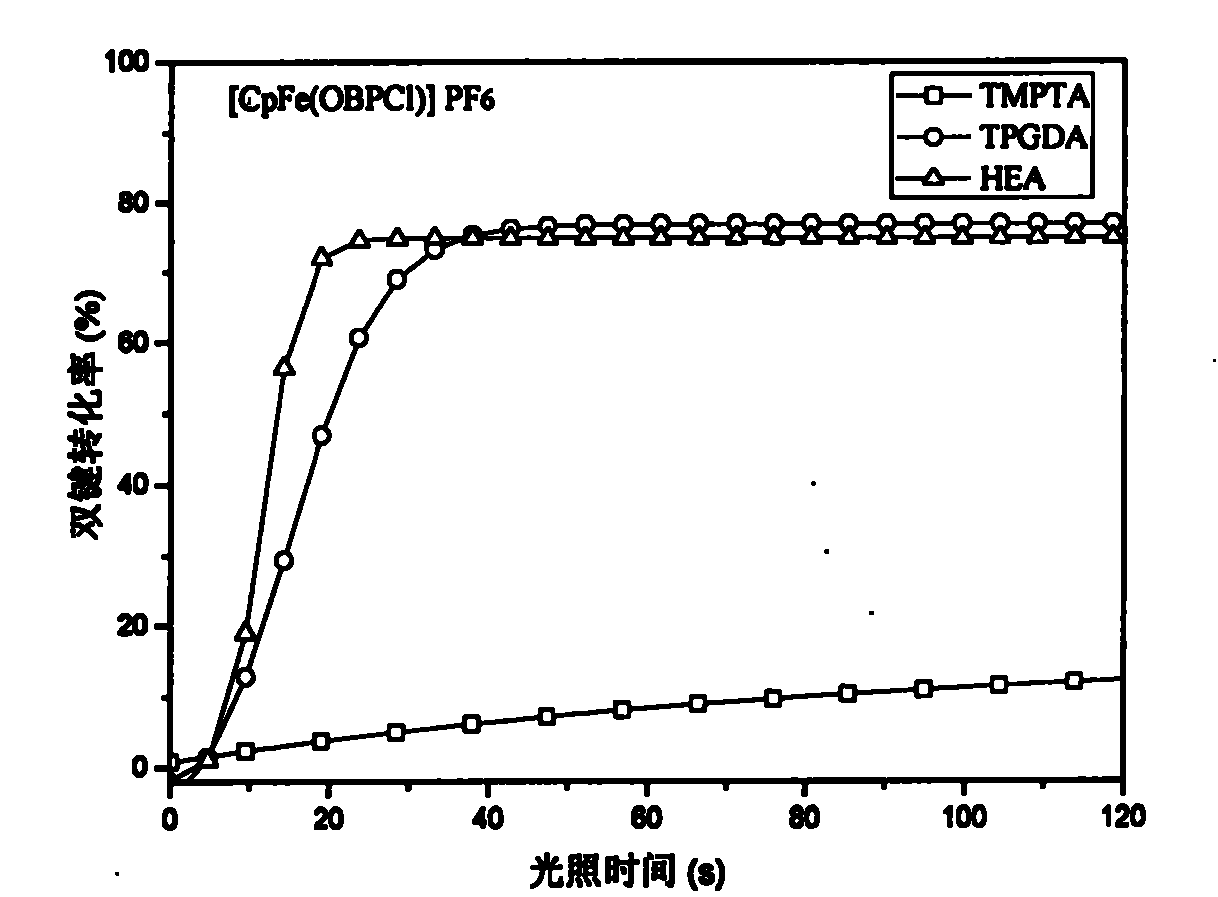
Image
Examples
Embodiment 1
[0031] [Cyclopentadiene-iron-(4-benzoylphenoxy)benzene]hexafluorophosphate ([CpFe(OBP)]PF 6 )Synthesis
[0032] The reaction equation is as follows:
[0033]
[0034] Add 10mmol (3.79g) ferrocene salt, 10mmol (1.98g) 4-hydroxybenzophenone, 50mlDMF and excess potassium carbonate 2g to a 100ml three-necked round bottom flask, start stirring, stir under nitrogen protection, and react For 4 hours, the temperature was kept at 60-80 degrees, and the reaction process was monitored by TCL. After the reaction, filter the reaction mixture, add 10% hydrochloric acid, adjust to neutral, pour into 300ml of ice water, add concentrated ammonium hexafluorophosphate solution, and obtain a yellow granular solid complex. Extract with dichloromethane, then wash with water for 5 times, add magnesium sulfate to dry, filter, remove the solvent by rotary evaporation, then dissolve with dichloromethane, and finally add dropwise into diethyl ether to obtain a yellow solid, filter, and then go thro...
Embodiment 2
[0036] Synthesis of [Cyclopentadiene-Iron-(4-(4'-chlorobenzoyl)phenoxy)benzene]hexafluorophosphate [CpFe(OBPCl)]PF6
[0037] The response formula is as follows:
[0038]
[0039]Add 1mmol (3.79g) ferrocene salt, 1mmol (2.32g) 4-hydroxyl-4'-chlorobenzophenone, 10mlDMF and excess potassium carbonate 2g to a 100ml three-neck round bottom flask, start stirring, and Stir under protection, react for 4 hours, control the temperature at 60-80 degrees, and monitor the reaction process with TCL. After the reaction, filter the reaction mixture, add 10% hydrochloric acid, adjust to neutral, pour into 300ml of ice water, add concentrated ammonium hexafluorophosphate solution, and obtain a yellow granular solid complex. Extract with dichloromethane, then wash with water 5 times, add magnesium sulfate to dry, filter, remove the solvent by rotary evaporation, then dissolve with dichloromethane, and finally add it dropwise to diethyl ether to obtain the final product [cyclopentadiene-iron-...
Embodiment 3
[0041] [Cyclopentadiene-Iron-N-(2-chloro-4-benzoyl)anilide]hexafluorophosphate [CpFe(NBPCl)]PF 6 Synthesis
[0042] The reaction equation is as follows:
[0043]
[0044] Add 1mmol (3.79g) ferrocene salt, 1mmol (2.31g) 2-amino-5-chlorobenzophenone, 10mlDMF and 1g excess sodium hydride to a 100ml three-necked round-bottomed flask, start stirring, and place under nitrogen protection Stirring was carried out, and the reaction process was monitored by TCL. After the reaction, filter the reaction mixture, add 10% hydrochloric acid, adjust to neutral, pour into 300ml of ice water, add concentrated ammonium hexafluorophosphate solution, and obtain a yellow granular solid complex. Extract with dichloromethane, then wash with water 5 times, add magnesium sulfate to dry, filter, rotary evaporate to remove the solvent, then dissolve with dichloromethane, and finally add it dropwise to ether to obtain the final product [cyclopentadiene-iron-N- (2-Chloro-4-benzoyl) aniline] hexafluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com