Organic flourescent material derived from glutamic acid
A technology of fluorescent materials and glutamic acid, applied in the fields of luminescent materials, organic chemistry, zinc organic compounds, etc., can solve the problems of harsh use conditions, difficult to obtain blue light, few types, etc., and achieve good thermal stability, high product purity, Easy to prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
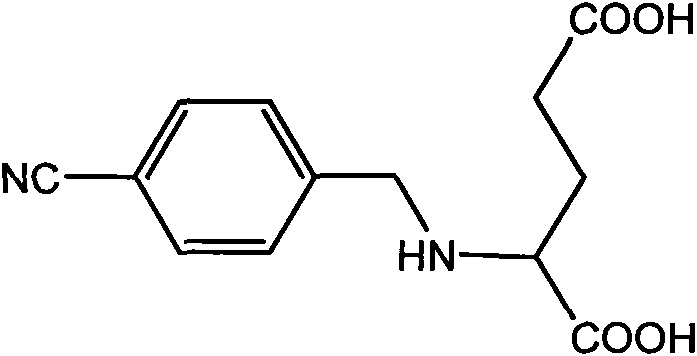
[0017] Synthesis of organic ligands: (S)-glutamic acid (11.7g, 0.08mol) was dissolved in 2M NaOH aqueous solution (0.16mol, 80mL), and 4-cyanobenzaldehyde (10.5g, 0.08mol) was added dropwise Ethanol (40 mL) solution. After dropping, the mixture was cooled to below 5°C with an ice bath, and NaBH was added in batches. 4 (1.0 g, 0.025 mol) to keep the temperature of the reaction solution at 0-5°C. After the addition, the reaction solution was raised to room temperature and stirred for 1.5 h. A solution of 4-cyanobenzaldehyde (2.1 g, 0.016 mol) in ethanol (10 mL) was added dropwise, followed by NaBH 4 (0.2g, 4.8mmol), the reaction solution was stirred for 0.5h, and unreacted 4-cyanobenzaldehyde was extracted with ether. The aqueous layer was separated, and hydrochloric acid was added dropwise at 0-5°C to adjust the pH to 3. At this time, a large amount of white solid precipitated out. Suction filter, wash the solid with ice water, and then dry the solid in a vacuum oven at 50...
Embodiment 2
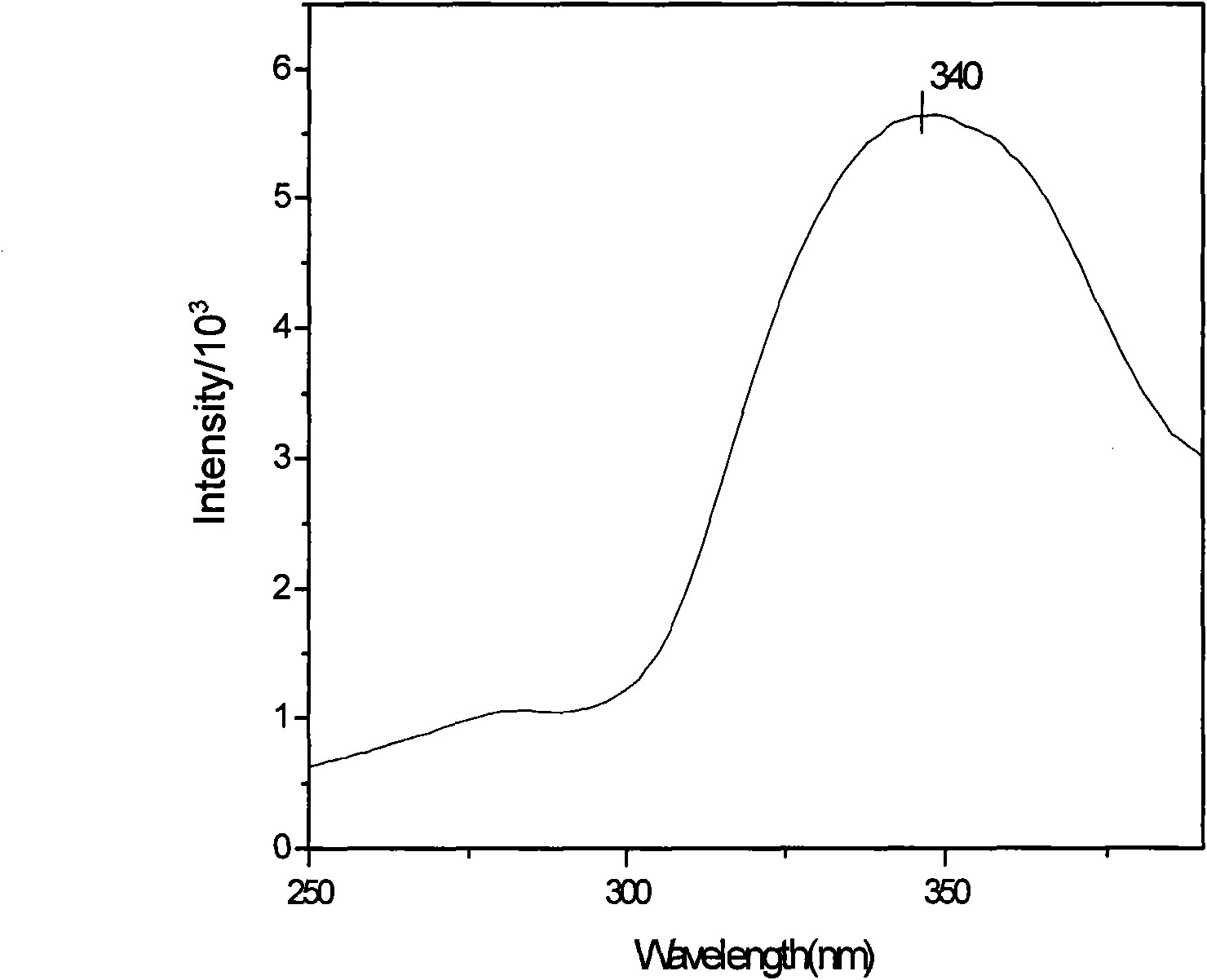
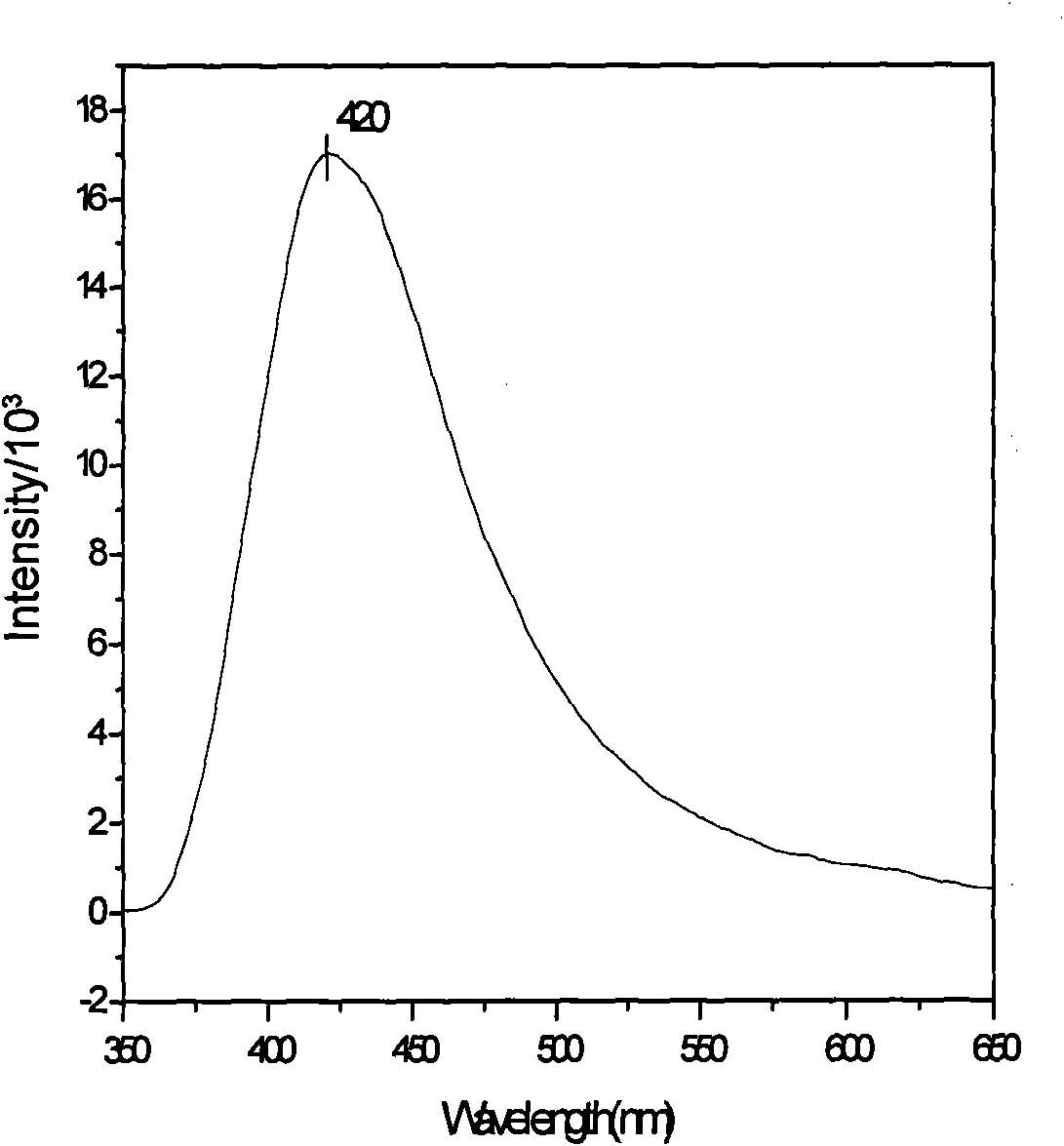
[0019] Synthesis of zinc complexes: In a small test tube, add zinc acetate aqueous solution (43.1mg, 0.5mmol / 5mL), then cover the NaOH aqueous solution (0.25mmol / 1mL) of ligand NC-Glu (65.6mg, 0.25mmol), place The mixed solution was heated to 60° C., and then slowly cooled to obtain 29.3 mg (32.4%) of colorless transparent needle-like microcrystals. Elemental analysis theoretical value (experimental value) %: C, 45.44 (44.87); H, 4.11 (4.07); N, 8.15 (8.20). Infrared spectrum (KBr, cm -1 ): 3300.09(m), 2225.32(m), 1609.74(s), 1567.53(s), 1391.18(m), 1250.41(w), 899.21(w). The compound emits blue fluorescence under 330nm ultraviolet light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com