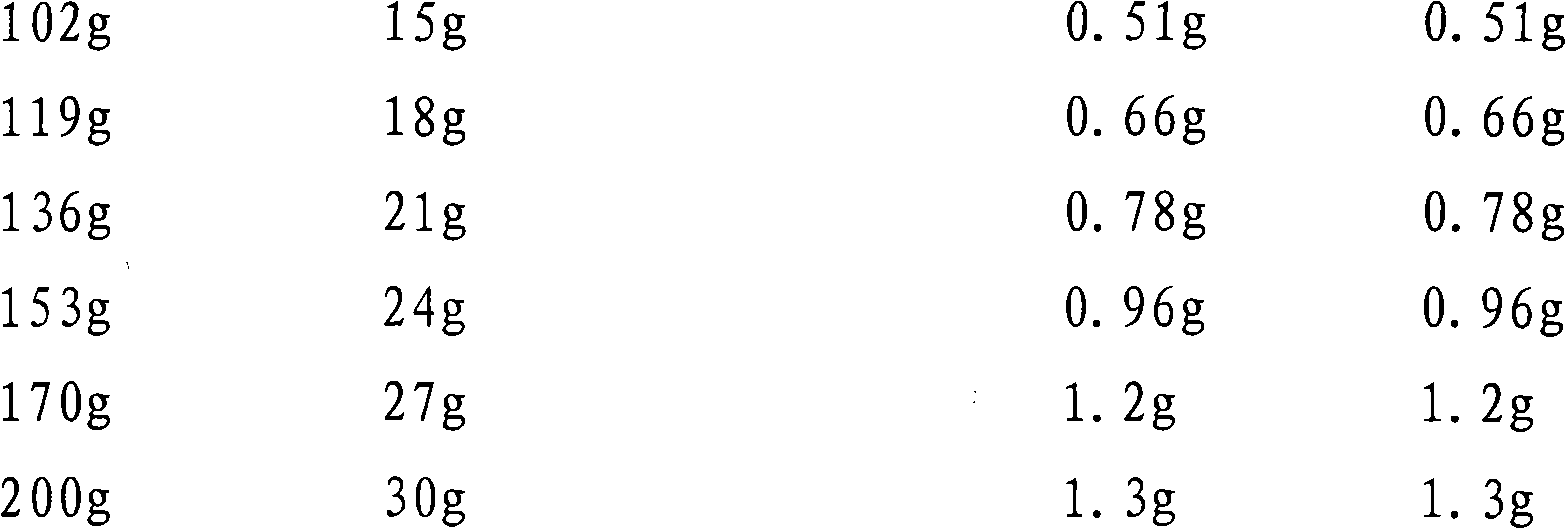Pharmaceutical composition for treatment of abnormal energy metabolism and application thereof
An energy metabolism and composition technology, which is applied in the fields of drug combination, metabolic diseases, and medical preparations containing active ingredients, etc., can solve the problems of fatty acid and glucose metabolism imbalance, no drug energy metabolism, mitochondrial dysfunction, etc. Rehabilitation, treatment of mitochondrial dysfunction, the effect of optimizing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
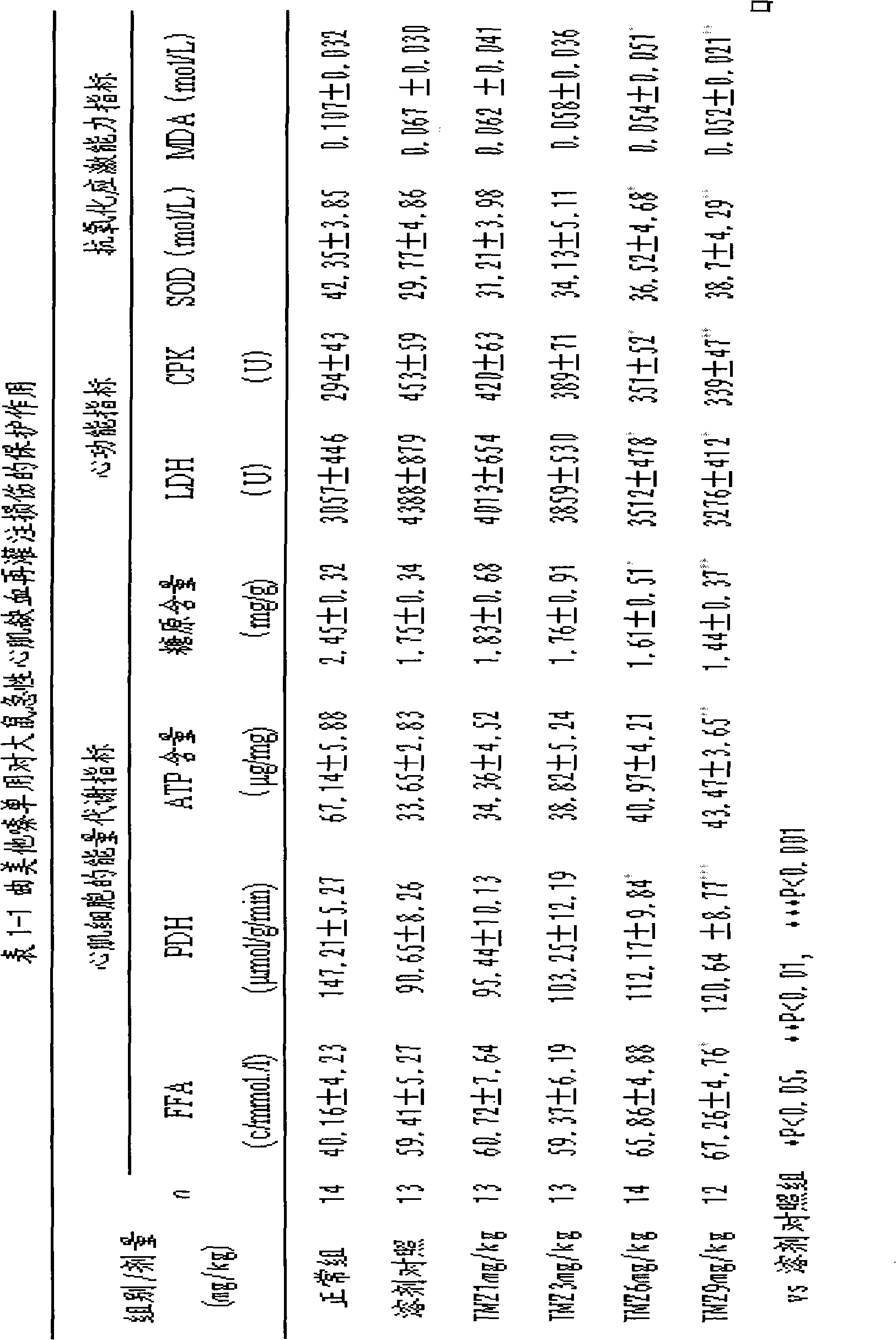
[0048] Example 1 The curative effect of trimetazidine and levocarnitine pharmaceutical composition on acute myocardial ischemia-reperfusion injury in rats, the curative effect on abnormal energy metabolism caused by myocardial ischemia, and the effect on the comprehensive balance of fatty acid and glucose metabolism , and efficacy against mitochondrial dysfunction.
[0049] During acute ischemia and reperfusion of the myocardium, due to insufficient oxygen, the energy supply of the myocardium changes from aerobic oxidation of fatty acids to glycolysis to provide energy. Therefore, a large amount of lactic acid produced by glycolysis leads to accumulation of acid products, A series of pathological changes such as aggravated peroxidation and damage to myocardial ultrastructure.
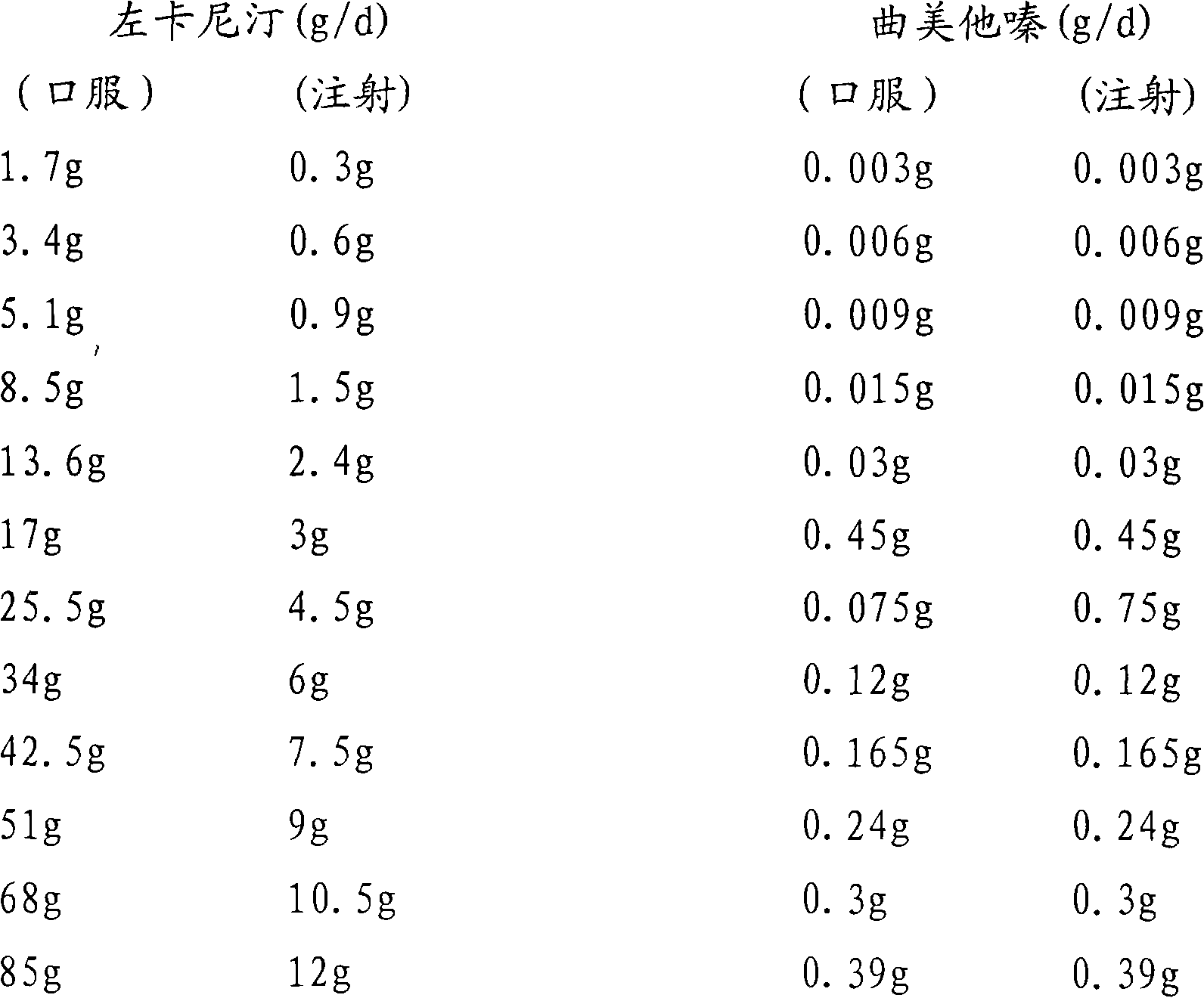
[0050] In order to observe the prevention and treatment effects of trimetazidine, levocarnitine and different ratios on acute myocardial ischemia-reperfusion injury, abnormal energy metabolism, and mito...
Embodiment 2
[0092] Example 2 Efficacy of Levocarnitine and Trimetazidine Composition in Treating Metabolic Syndrome and Abnormal Energy Metabolism
[0093] 2.1 Modeling method:
[0094] Animals: ordinary male Wistar rats, body weight 200±20g; after feeding with ordinary feed for 1 week, a) each model group was given high-sugar, high-fat, and high-salt feed (basic feed 58%, lard oil 8%, sucrose 22% , egg yolk 10%, salt 2%) to detect modeling indexes after 8 weeks. b) Normal control group: fed with common feed (containing 0.5% salt) for 8 weeks. All animals had free access to food and water
[0095] 2.2 Test drugs and grouping
[0096] 1) Drugs: Levocarnitine and trimetazidine hydrochloride were provided by Changzhou Sanwei Industrial Research Institute.
[0097] Solvent: normal saline. The test drug was prepared with normal saline to make an equal volume solution of corresponding concentration before use.
[0098] 2) Dose setting and experimental grouping
[0099] After the equivale...
Embodiment 3
[0129] Example 3 Efficacy of Levocarnitine and Trimetazidine Composition in Treating Abnormal Energy Metabolism of Liver Cancer
[0130] 3.1 Experimental method and dose setting
[0131] BALB / c mice, male. Weight 20-22g. 15 mice were randomly selected from each batch of 36 mice as the healthy group.
[0132] The remaining groups were subcutaneously injected with HepA cell suspension in the right forelimb, each 0.2mL (5x10 / ml) to prepare liver cancer samples.
[0133] Body tumor model. After the equivalent doses of trimetazidine and levocarnitine were calculated based on clinical human doses, each
[0134] There are 2 dosage groups, 4 groups including solvent group and control group. They are:
[0135] Trimetazidine (TMZ) group: solvent control group, TMZ 48mg / kg administration group, intraperitoneal injection.
[0136] L-carnitine (L-C) group: solvent control group, L-C 340mg / kg administration group, intragastric administration.
[0137] Trimetazidine + levocarnitine d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com