Method for synthesizing fluorescent silver nano-particles under microwave by polymethylacrylic acid
A technology of polymethacrylic acid and sodium polymethacrylate, applied in the field of fluorescent nano silver particles, can solve the problems of tedious and time-consuming operation, and achieve the effects of uniform particle size distribution, good dispersibility and easy realization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Measure 4.8mL (0.05M) AgNO with a pipette 3 The aqueous solution is placed in a 20mL beaker, 0.6mL of polymethacrylic acid aqueous solution (molecular weight 5,000) is added, the two are mixed uniformly, the pH is adjusted to 82 with hydrochloric acid or NaOH, placed in an 8mL quartz reaction flask, and the cap is sealed. The reaction flask was put into the microwave heating cavity, and then treated in a microwave reactor at 120°C for 30 minutes. After the reaction system is naturally cooled to room temperature, it is stored in a 4°C low-temperature refrigerator and protected from light.
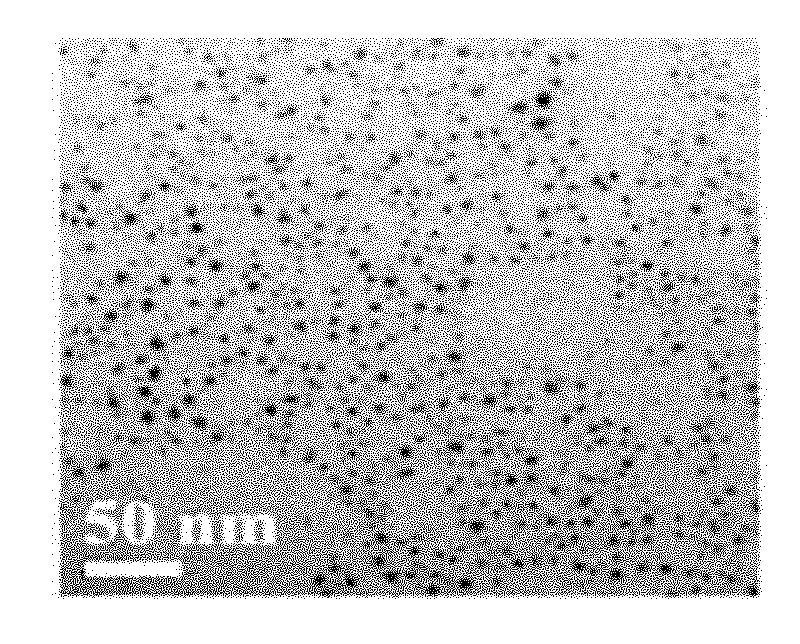
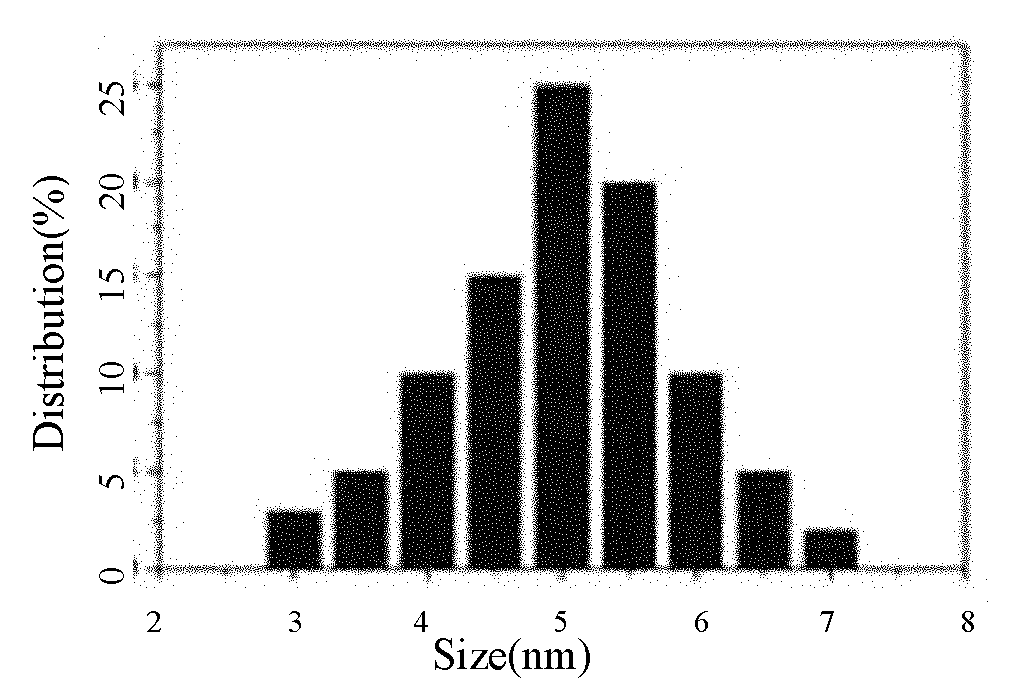
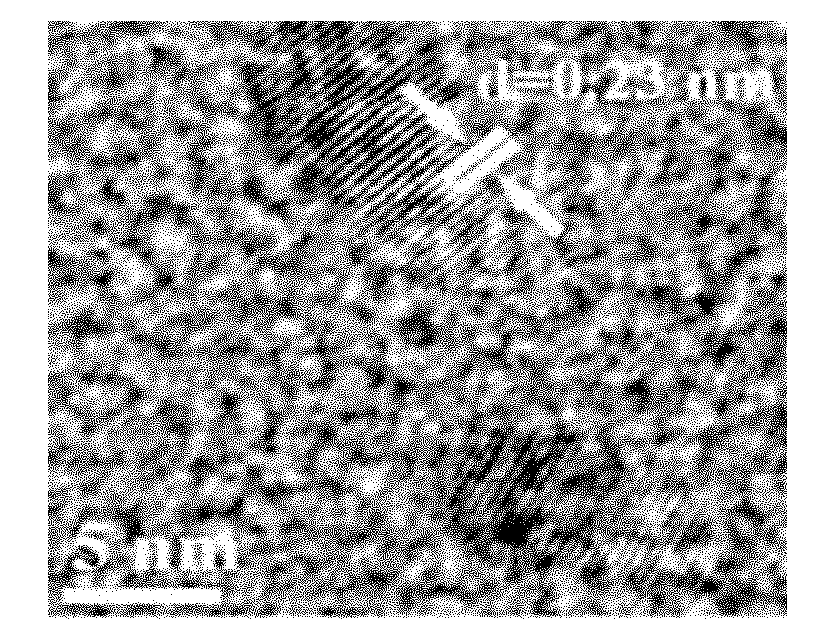
[0035] For the TEM image of the silver nanoparticles prepared in Example 1, see figure 1 For the particle size distribution diagram of the silver nanoparticles prepared in Example 1, see figure 2 For the high-resolution TEM image of the silver nanoparticles prepared in Example 1, see image 3 For the selected area electron diffraction pattern of the silver nanoparticles prepared in Examp...
Embodiment 2
[0036] Example 2 Influence of PMAA concentration on fluorescence intensity
[0037] Measure 3mL (0.05M) AgNO with a pipette 3 The aqueous solution, placed in a 20mL beaker, add 0.3mL of polymethacrylic acid aqueous solution (molecular weight 10,000) and 0.4mL of polymethacrylic acid aqueous solution (molecular weight 50,000), mix the three evenly, adjust the pH to 8.0 with hydrochloric acid or NaOH , Placed in an 8mL quartz reaction flask, sealed with a cap, put the reaction flask into a microwave heating chamber, and then treated in a microwave reactor at 120°C for 30 minutes. After the reaction system is naturally cooled to room temperature, the fluorescence spectrum is measured at the maximum fluorescence excitation wavelength of 515 nm.
[0038] For the fluorescence spectrum of the silver nanoparticles prepared in Example 2, see Figure 5 .
Embodiment 3
[0039] Example 3 The influence of different pH on fluorescence intensity
[0040] Measure 4.8mL (0.05M) AgNO with a pipette 3 Aqueous solution, placed in a 20mL beaker, add 0.8mL sodium polymethacrylate aqueous solution (molecular weight 100,000), mix the two evenly, adjust the pH to 3.0~8.5 with hydrochloric acid or NaOH, put it in an 8mL quartz reaction flask, and press the cap Seal, put the reaction flask into the microwave heating cavity, and then treat it in a microwave reactor at 120°C for 30 minutes. After the reaction system is naturally cooled to room temperature, the fluorescence spectrum is measured at the maximum fluorescence excitation wavelength of 515 nm.
[0041] For the fluorescence spectrum of the silver nanoparticles prepared in Example 3, see Image 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com