Method for producing high-purity donepezil hydrochloride anhydrous I type crystal form and product thereof
A technology of donepezil hydrochloride and a production method, applied in the field of pharmaceutical synthesis, can solve problems such as low residual amount, unsolved moisture content, etc., and achieve the effects of simple production method, few steps, and overcoming technical barriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
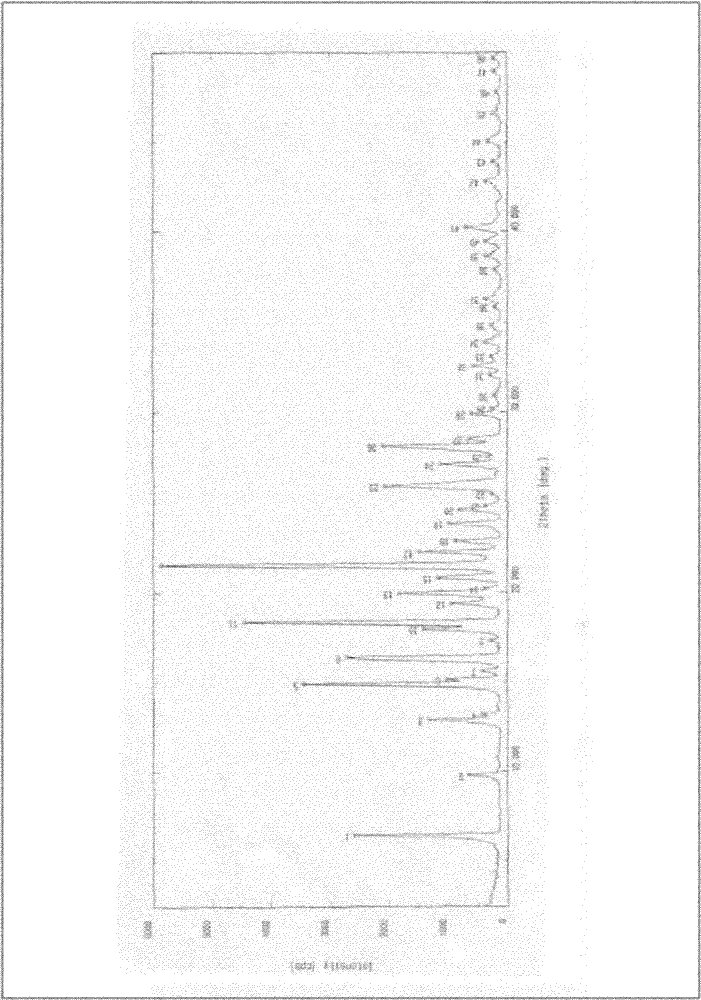
[0029] According to the prior art (publication number: CN101723878A), 35.0 g of white solid donepezil free base was prepared. Dissolve in 140.0 g of toluene, add 10 ml of concentrated hydrochloric acid, heat to reflux until the internal temperature reaches 111 ° C, cool to room temperature, filter the precipitated crystals, and dry to obtain 37.0 g of crude crystalline form of donepezil hydrochloride III, with a yield of 96.4%. Its X-ray diffraction pattern is as figure 1 shown.
[0030] According to the above preparation, 30.0 g of crude product of donepezil hydrochloride in crystal form II was obtained. Suspended in 120.0 g of methanol, 1.3 g of pure water was added. The solution was heated and stirred to make the solution clear, and then cooled to room temperature, crystals were precipitated, further cooled to -10°C, and kept at this temperature for 3 hours for crystallization.
[0031] The precipitated crystals were filtered off. Put it into a vacuum oven, maintain the...
Embodiment 2
[0033] According to the prior art (publication number: CN101723878A), 35.0 g of white solid donepezil free base was prepared. Dissolve in 200.0 g of toluene, add 20 ml of concentrated hydrochloric acid, heat to reflux until the internal temperature reaches 111° C., cool to room temperature, filter the precipitated crystals, and dry to obtain 36.4 g of crude crystal form of donepezil hydrochloride III, with a yield of 94.8%.
[0034] According to the above preparation, 30.0 g of crude product of donepezil hydrochloride in crystal form III was obtained. Suspended in 200.0 g of methanol, 4.0 g of pure water was added. The solution was heated and stirred to make the solution clear, and then cooled to room temperature to precipitate crystals, further cooled to -5°C, and kept at this temperature for 4 hours to crystallize.
[0035] The precipitated crystals were filtered off. Put it into a vacuum oven, maintain the vacuum at -0.95MPa, and bake at 45°C for 24 hours. Under the prot...
Embodiment 3
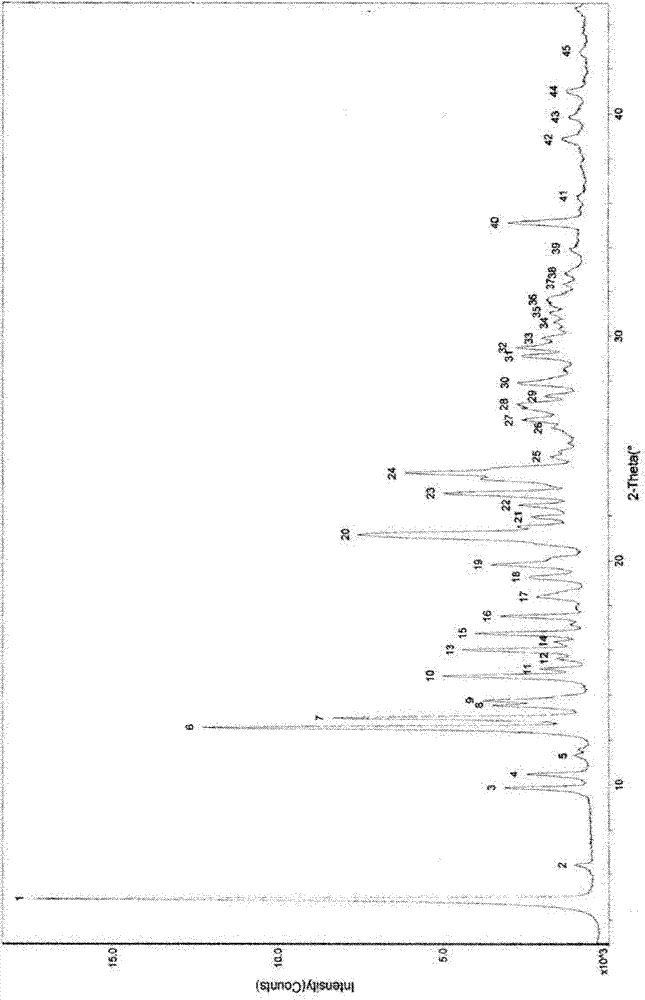
[0037] According to the method of the prior art (publication number: CN1221404A), 30.0 g of donepezil hydrochloride crystal form I compound was prepared, with a water content of 5.22%. 150.0 g methanol containing 0.15 g water was added. The solution was heated and stirred to make the solution clear, and then cooled to room temperature to precipitate crystals, further cooled to -10°C, and kept at this temperature for 4 hours to crystallize. The precipitated crystals were filtered off. Put it in a vacuum oven, keep the vacuum at -0.95MPa, bake at 45°C for 4 hours; then bake at -0.80MPa, 75°C for 8 hours. Under the protection of nitrogen, the vacuum was broken and cooled to room temperature to obtain 25.5 g of anhydrous I crystal form of donepezil hydrochloride. The content is 99.80%, the single impurity is less than 0.1%, and the moisture content is 0.19% measured by Karl Fischer moisture determination method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com