Preparation method of articaine hydrochloride
A technology for articaine hydrochloride and amidation, which is applied in the field of preparation of articaine hydrochloride, can solve the problems of cumbersome operation, harsh reaction conditions, and low reaction yield, and achieve high yield, simple operation, and low raw material Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
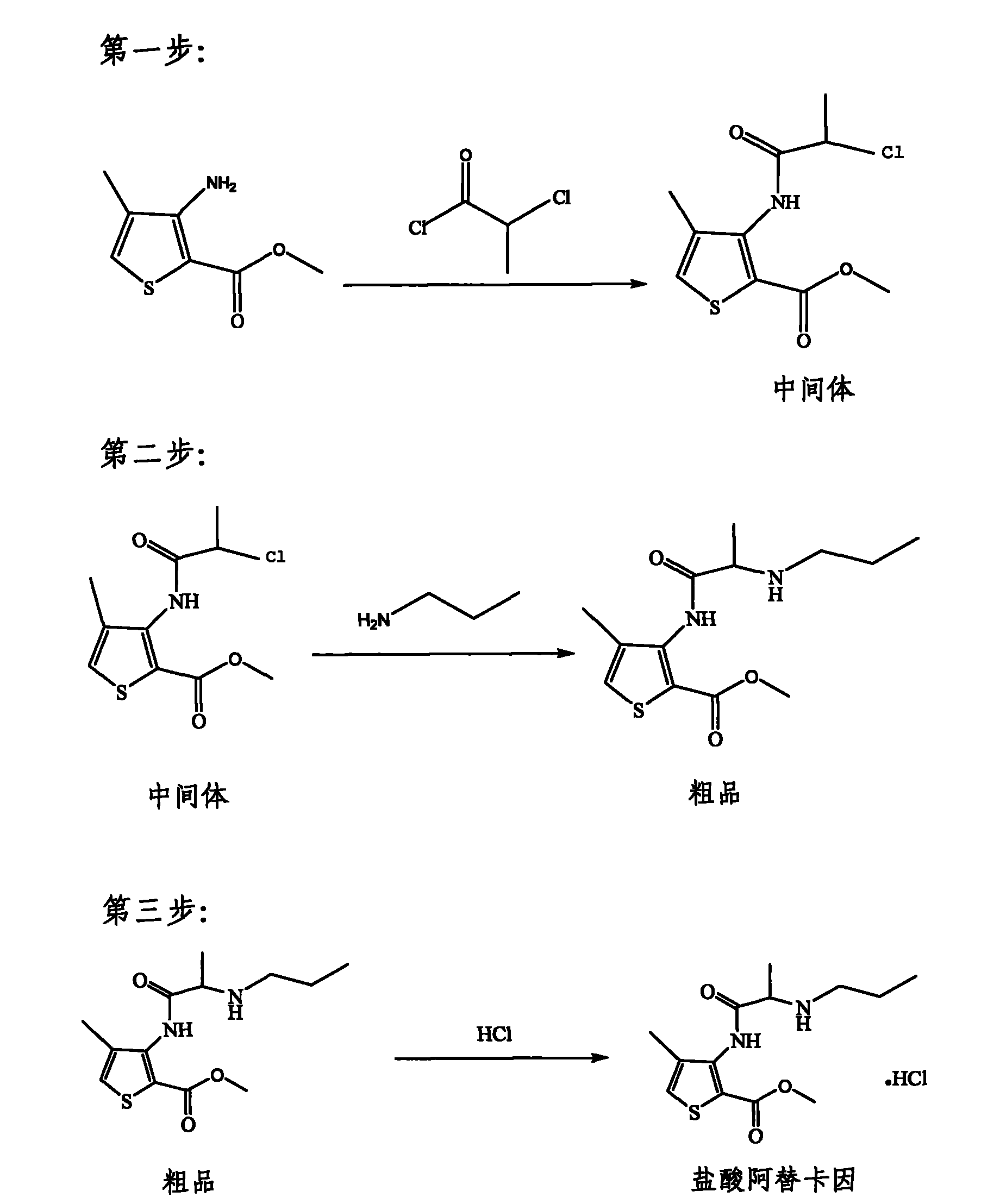
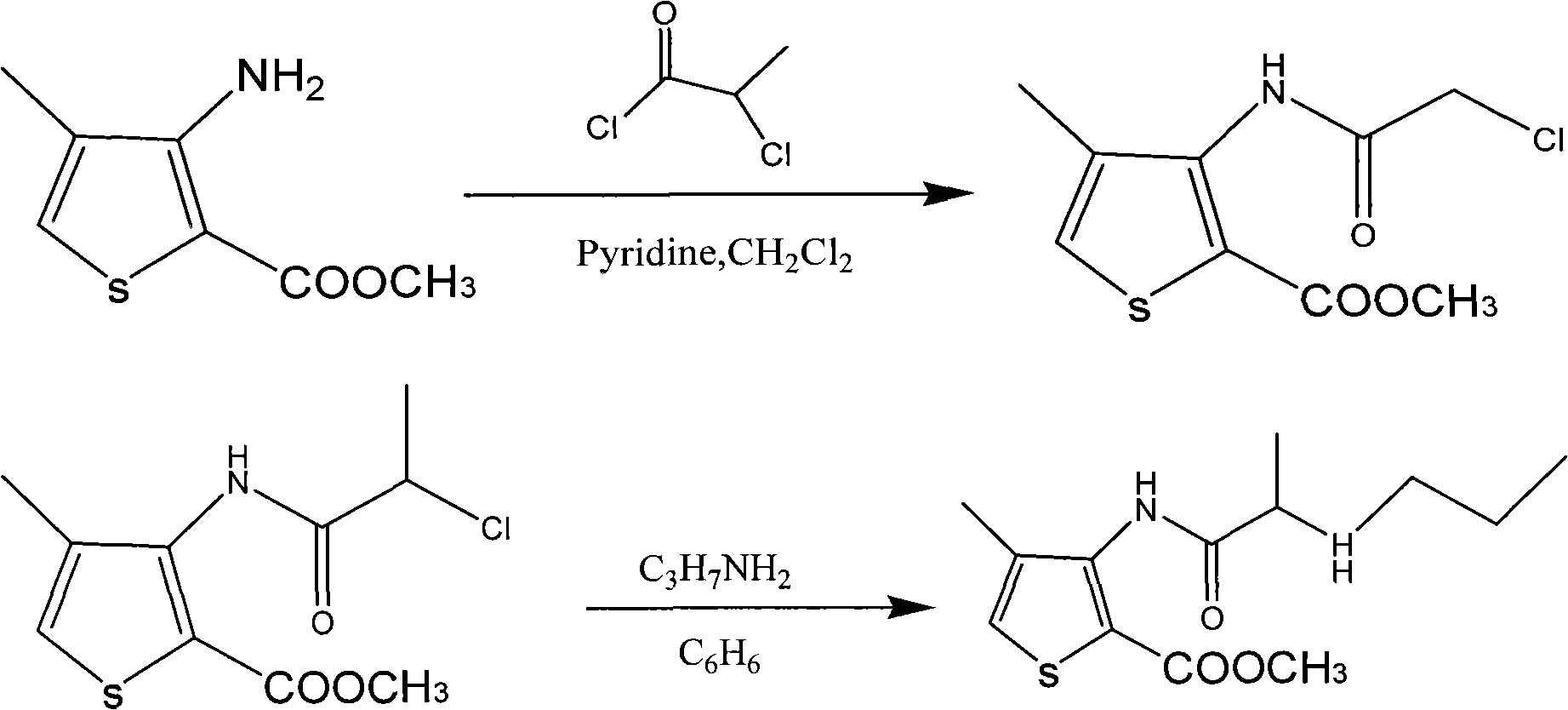
[0032] The first step: amidation of methyl 4-methyl-3-aminothiophene-2-carboxylate
[0033] Add 1600ml of dichloromethane into the reaction kettle, add 800g of 4-methyl-3-aminothiophene-2-carboxylic acid methyl ester (yellow) with a funnel, stir to dissolve the solid. Use an ice-water bath to lower the reaction kettle to about 10°C, add 700ml of triethylamine with stirring, and then slowly add 580ml of 2-chloropropionyl chloride dropwise, a large amount of white smoke and a large amount of white solids can be seen in the reaction vessel. After the dropwise addition was completed, the ice-water bath was naturally warmed up to room temperature, and reacted for 5 hours.
[0034] Slowly pour the resulting reaction solution into the barrel, add 1600ml of dichloromethane, add 1600ml of distilled water, stir mechanically, and carefully add Na 2 CO 3 Solid, to neutralize excess 2-chloropropionyl chloride, adjust the pH to be alkaline (pH 7.5-9). During this process, a large amount ...
Embodiment 2
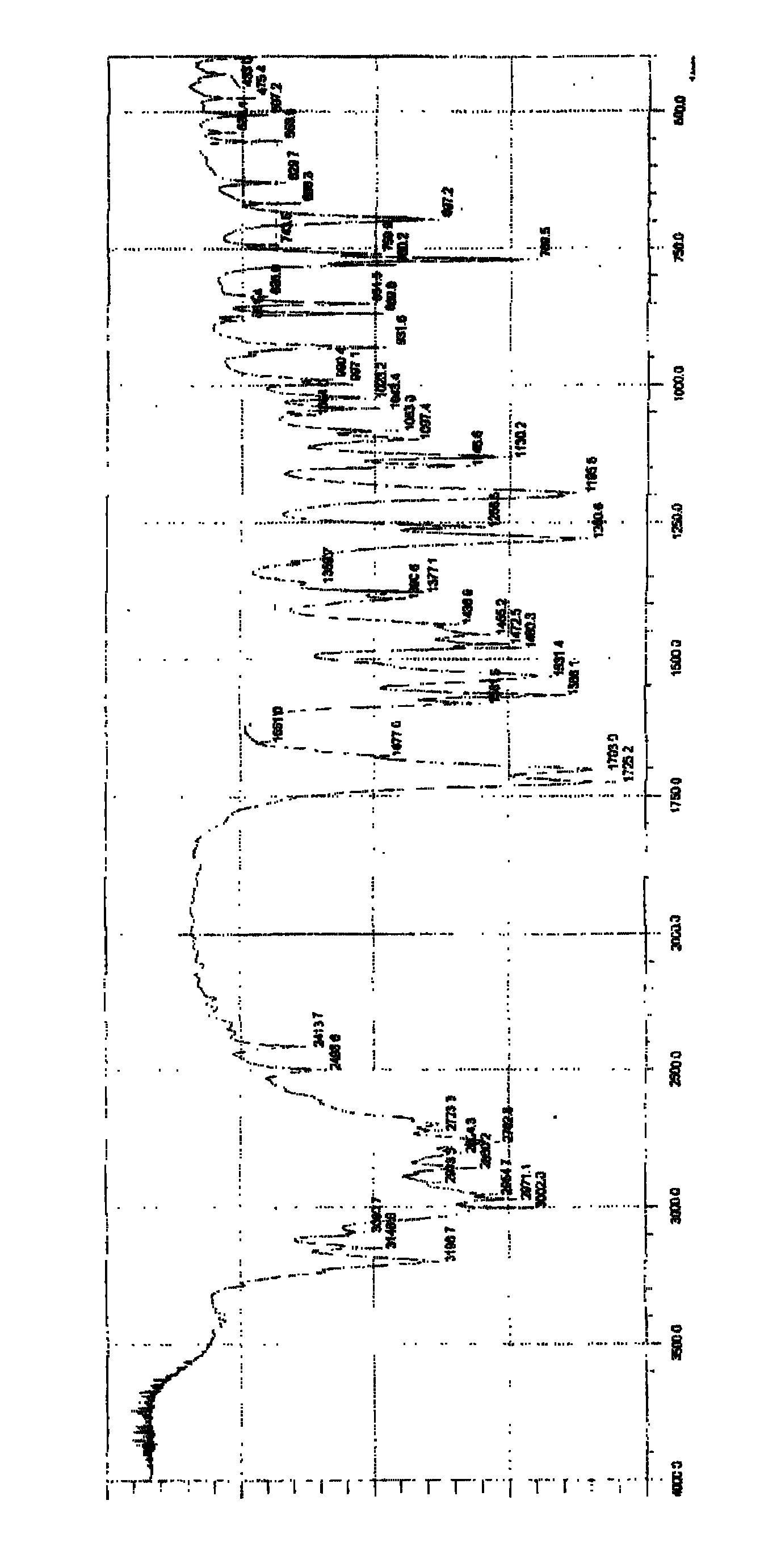
[0041] The same operation was performed as in Example 1, only 700 ml of triethylamine used in the amidation reaction step was replaced with 960 g of potassium carbonate, and the others were kept unchanged to obtain 850 g of intermediate, the yield of this step was 70%, and the melting range: 97-98 ° C. The reaction was continued to obtain 830 g of articaine oil, and the yield of this step was 90%. After salt formation, 795g of the final product was obtained, with a purity of 99.1%, and a yield of 85% in this step. The infrared spectrum of the product obtained is similar to the final product of Example 1.
Embodiment 3
[0043] The same operation was carried out as in Example 1, only the 2-chloropropionyl chloride used in the amidation reaction step was replaced with 2-chloropropionyl bromide, and the others were kept unchanged to obtain 1076 g of the intermediate. 1020 g of the final product was obtained, the melting range was 118-119° C., the purity was 99.3%, and the yield was about 85%. The infrared spectrum of the product obtained is similar to the final product of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com