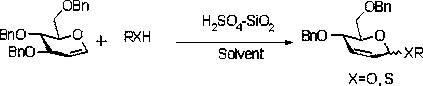Method for preparing 4, 6-dibenzyl-2, 3-unsaturated glucoside
An unsaturated and dibenzyl technology is applied in the field of preparation of 4,6-dibenzyl-2,3-unsaturated glycosides, which can solve the problems of easy corrosion of catalysts, narrow reaction scope, limited wide application, etc. To achieve the effect of cheap solvent, low toxicity, wide application range and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 0.1mmol 3,4,6-tri-O-benzyl-D-glucene 40mg and 1mL dichloromethane respectively in the reaction flask and mix, add 10mg sulfuric acid / silicon dioxide solid catalyst (H 2 SO 4 / SiO 2 ) and 0.5mmol methanol 0.02mL at 0 o The rearrangement reaction was carried out under the condition of C, and the reaction was tracked and monitored by TLC plate (PE:EA=10:1). After 0.5 hours of reaction, TLC monitored that the raw material was not completely converted, and 5 mg of sulfuric acid / silica solid catalyst was added. After 2 hours, the raw material After conversion, the rearrangement reaction of 3,4,6-tri-O-benzyl-D-glucocene is completed, the catalyst is filtered off, and the filtrate is concentrated to obtain a colorless viscous liquid which is 4,6-di-O-benzyl- D-glucosene methyl glycoside 15mg, yield 92%.
[0016] The NMR structure analysis of 4,6-di-O-benzyl-D-glucurene methyl glycoside, the test data is as follows :
[0017] 1 H NMR (500 MHz, CDCl 3 ): (α:β=8.5:1) ...
Embodiment 2
[0020] Add 0.5mmol 3,4,6-tri-O-benzyl-D-glucene 200mg and 10mL acetonitrile respectively in the reaction flask and mix, add 25mg sulfuric acid / silica solid catalyst (H 2 SO 4 / SiO 2 ) and 1.2mmol dehydroepiandrosterone 0.33g, at 50 o The rearrangement reaction was carried out under the condition of C, and the reaction was followed and monitored by TLC plate (PE:EA=10:1), and the reaction was monitored by TLC after 10 minutes. The catalyst was filtered off, and the filtrate was concentrated to obtain 0.25 g of pure white solid 4,6-di-O-benzyl α-D-glucosene dehydroepiandrosterone glycoside, with a yield of 87%. The deagent is petroleum ether: ethyl acetate=10:1~100:1 (v / v).
[0021] NMR structure analysis of 4,6-di-O-benzyl α-D-glucosene dehydroepiandrosterone glycoside, the test data are as follows :
[0022] 1 H NMR (500 MHz, CDCl 3 ): δ =7.35-7.23 (m, 10H), 6.09 (d, J =10.2Hz, 1H), 5.77 (m, 1H), 5.27 (m, 1H), 5.17(s, 1H), 4.67 (d, J =12.2Hz, 1H), 4.62 (d, J =11.5H...
Embodiment 3
[0025] Add 0.15mmol 3,4,6-tri-O-benzyl-D-glucoene 40mg and 1mL diethyl ether respectively in the reaction flask and mix, add 50mg sulfuric acid / silicon dioxide solid catalyst (H 2 SO 4 / SiO2 ) and 0.2mmol menthol 30mg, in 10 o The rearrangement reaction was carried out under the condition of C, and the reaction was tracked and monitored by TLC plate (PE:EA=10:1). After 50 minutes, TLC monitored the reaction to be complete, filtered off the catalyst, and concentrated the filtrate to obtain 3,4,6-tri-O-benzyl 45 mg of the rearrangement product of D-glucosene, the product was separated by silica gel column chromatography (100-200 mesh, PE:EA=25:1), and the pure colorless syrup 4,6-di-O-benzyl was obtained Base-D-glucosene menthol glycoside 40.2mg, yield 90%.
[0026] NMR structure analysis of 4,6-di-O-benzyl-D-glucosene menthol glycoside, the test data is as follows :
[0027] 1 H NMR (500 MHz, CDCl 3 ): (α:β=10:1) δ =7.35-7.23 (m, 10H), 6.07 (d, J =10.2Hz, 1H), 5.80-5.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com