Tumor specific target polypeptide and application thereof
A tumor-specific, peptide-targeting technology, applied to phage-displayed peptides and their application fields, can solve the problems of uncontrollable late-stage metastasis, drug resistance, unacceptability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1. Establishment of nude mouse model of human A460 large cell lung cancer
[0071] 1) Take four-week-old Balb / C female nude mice (purchased from the Experimental Animal Center of Beijing Academy of Medical Sciences) and put them into an SPF grade animal room for breeding.
[0072] 2) Cultivate human non-small cell lung cancer NCI H460, take the logarithmic growth phase lung cancer cell line, digest with trypsin routinely, and adjust the cell concentration to 1x10 7 pcs / ml, take 200ul that is 2x10 6 Cells were inoculated subcutaneously under the armpit of the forelimb of each nude mouse, and the growth status of the cells after inoculation was observed. After about 5-6 days, a tumor mass was visible. After two weeks, the diameter of the tumor was about 0.3-0.5 cm, which can be used for phage screening in vivo experiment.
[0073] 2. In vivo screening of phage display peptide library in mice
[0074] (1) Culture of E.coli ER2738
[0075] Pick an inoculation loop from ...
Embodiment 2
[0120] Chemical Synthesis and Identification of Cyclic Heptapeptide:
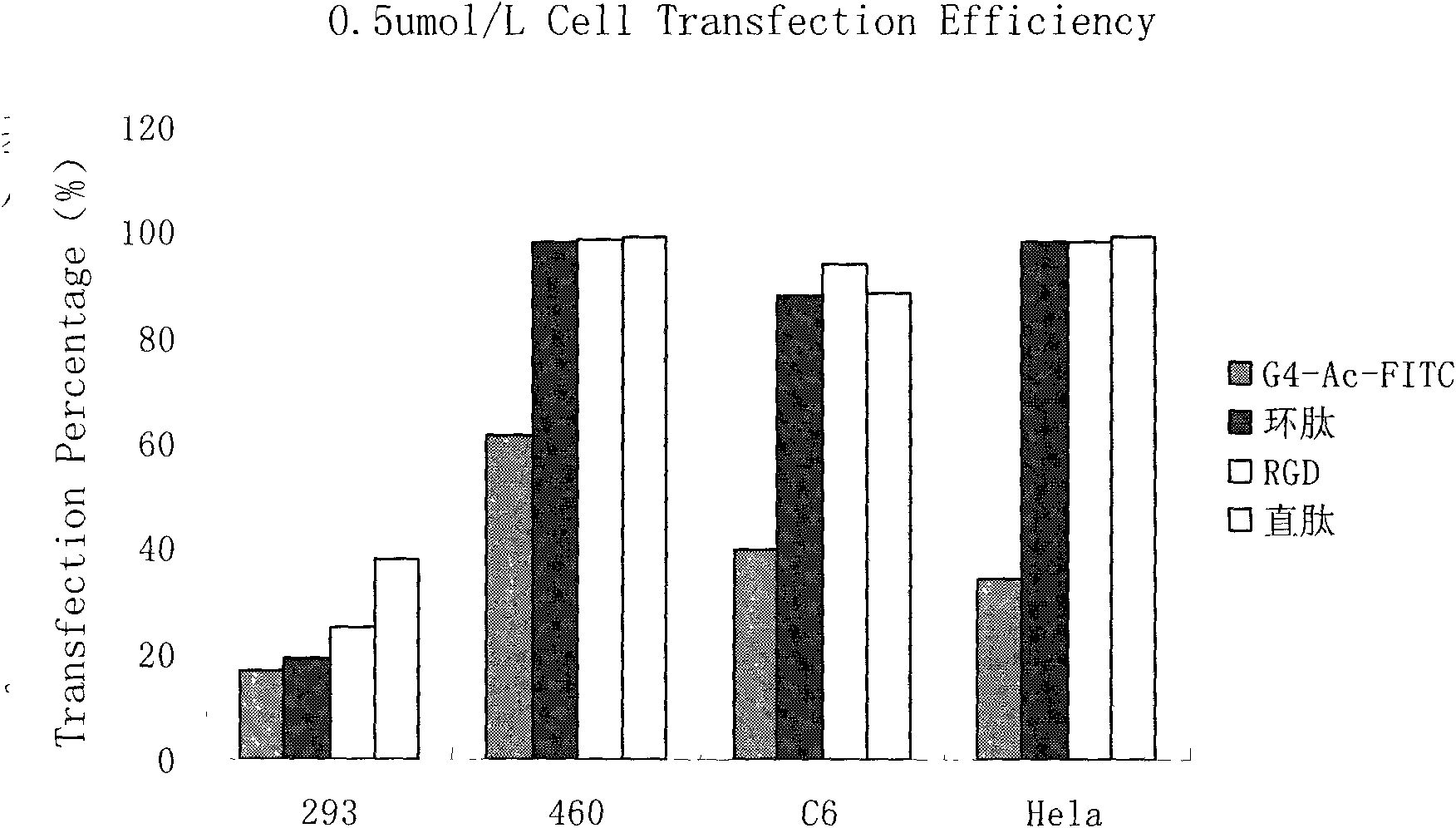
[0121] From the results of the sequencing of the cyclic heptapeptide, the amino acid sequence of the cyclic heptapeptide was deduced to be ACPLSHSLIC. .The PLSHSLI in the middle of CPLSHSLIC plays a targeting role. Synthesized and labeled with FITC (fluorescein isothiocyanate) as the detection material. The cyclic heptapeptide was purified and identified by HPLC. The results showed that the synthetic cyclic heptapeptide had a purity of more than 98%, and its molecular weight was consistent with the theoretical value.
Embodiment 3
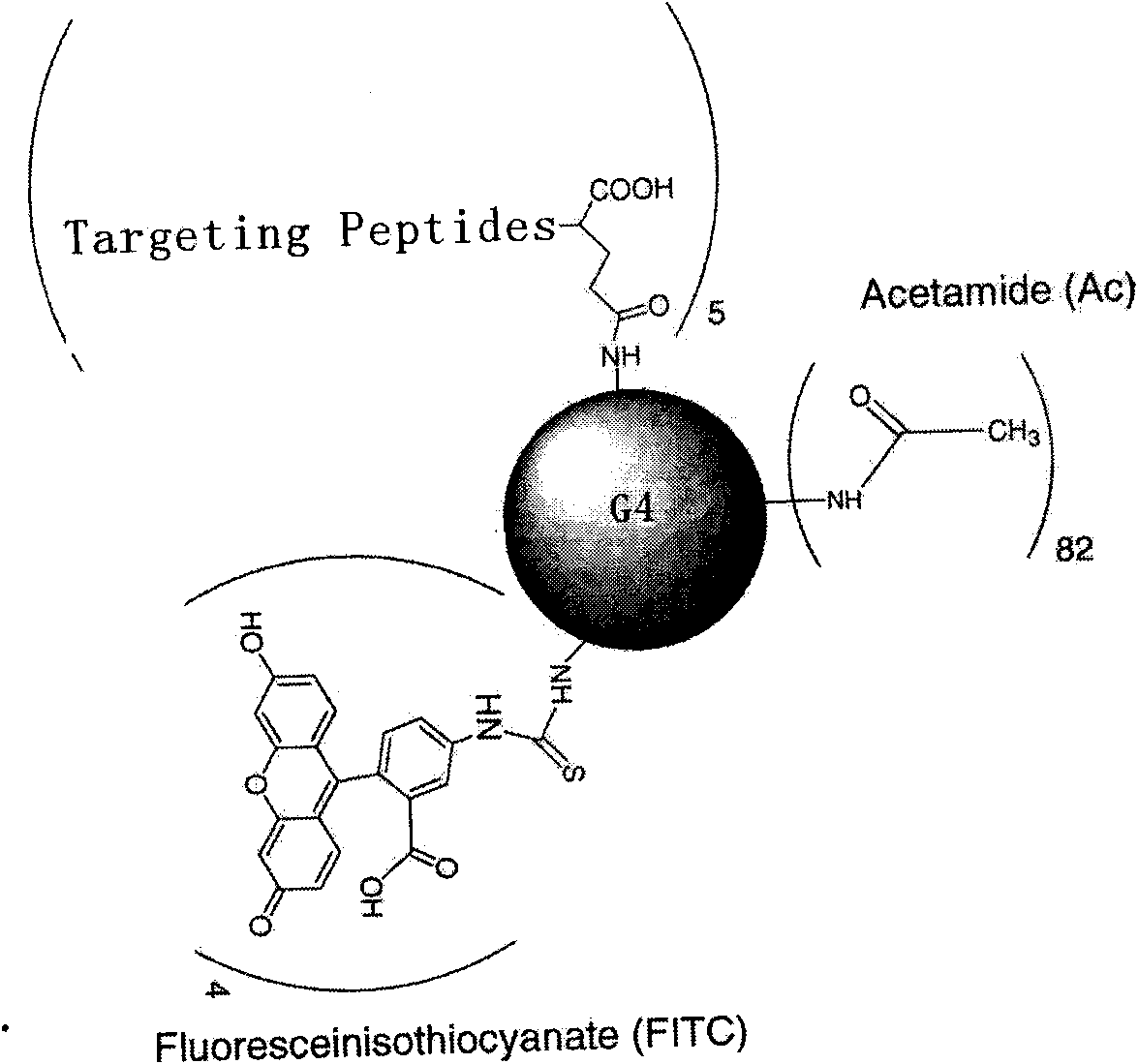
[0123] The polypeptide is connected with the nanomaterial polyamide-amine dendrimer (PAMAM) by chemical cross-linking method, and the PAMAM is connected with fluorescein isothiocyanate (FITC) as a detection label.
[0124] 1. Acetylation of fourth-generation PAMAM
[0125] Dissolve 1 g of PAMAM in 120 ml of anhydrous methanol, and add 0.35 g of triethylamine. Dissolve 0.21g of acetyl anhydride in 60ml of anhydrous methanol, and add dropwise to the above solution, and stir overnight at room temperature. Anhydrous methanol was evaporated in rotary vacuum and the residue was dissolved in water. Dialyze through a dialysis bag to remove unreacted small molecular substances, and freeze-dry after dialysis.
[0126] 2. Connect to FITC
[0127] Weigh 0.4 g of the acetylated PAMAM and dissolve in 40 ml of DMSO, dissolve 38.9 mg of FITC in 8 ml of DMSO, add dropwise to the PAMAM solution, and stir overnight. The reaction solution was dialyzed to remove unreacted small molecular subst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com