N-alkylamino-2-perfluoroalkylimidazoline quaternary ammonium salt and preparation method thereof
A technology of perfluoroalkylimidazoline and fluorocarbon imidazoline, which is applied in the field of N-alkylamino-2-perfluoroalkylimidazoline quaternary ammonium salt and its preparation, can solve the problem of sulfate-reducing bacteria without bactericidal performance and containing High cost of fluorosurfactants, ineffective corrosion inhibition and other problems, to achieve high thermodynamic stability and chemical stability, low pollution of three wastes, and not easy to hydrolyze
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 500ml stainless steel reactor equipped with a thermometer, agitator and pressure gauge, add 232g of perfluorononanoic acid, heat to 70°C and stir evenly, then add 78g of diethylenetriamine, add 1g of mesoporous alumina catalyst, and seal the reactor. The system was heated to 120°C, reacted for 8 hours under medium speed stirring, and cooled to room temperature within 2 hours without separation, directly added 64g of benzyl chloride and then heated to 100°C, and reacted for 10 hours under low speed stirring to obtain N-ethylamino-2- Perfluorononyl imidazoline quaternary ammonium salt. The N-ethylamino-2-perfluorononyl imidazoline quaternary ammonium salt structural formula that the present embodiment makes is shown in (1) (2), wherein R 1 for CF 3 -(CF 2 ) 8 , R 2 Ethyl N-alkylamino-2-perfluoroalkyl imidazoline quaternary ammonium salt.
Embodiment 2
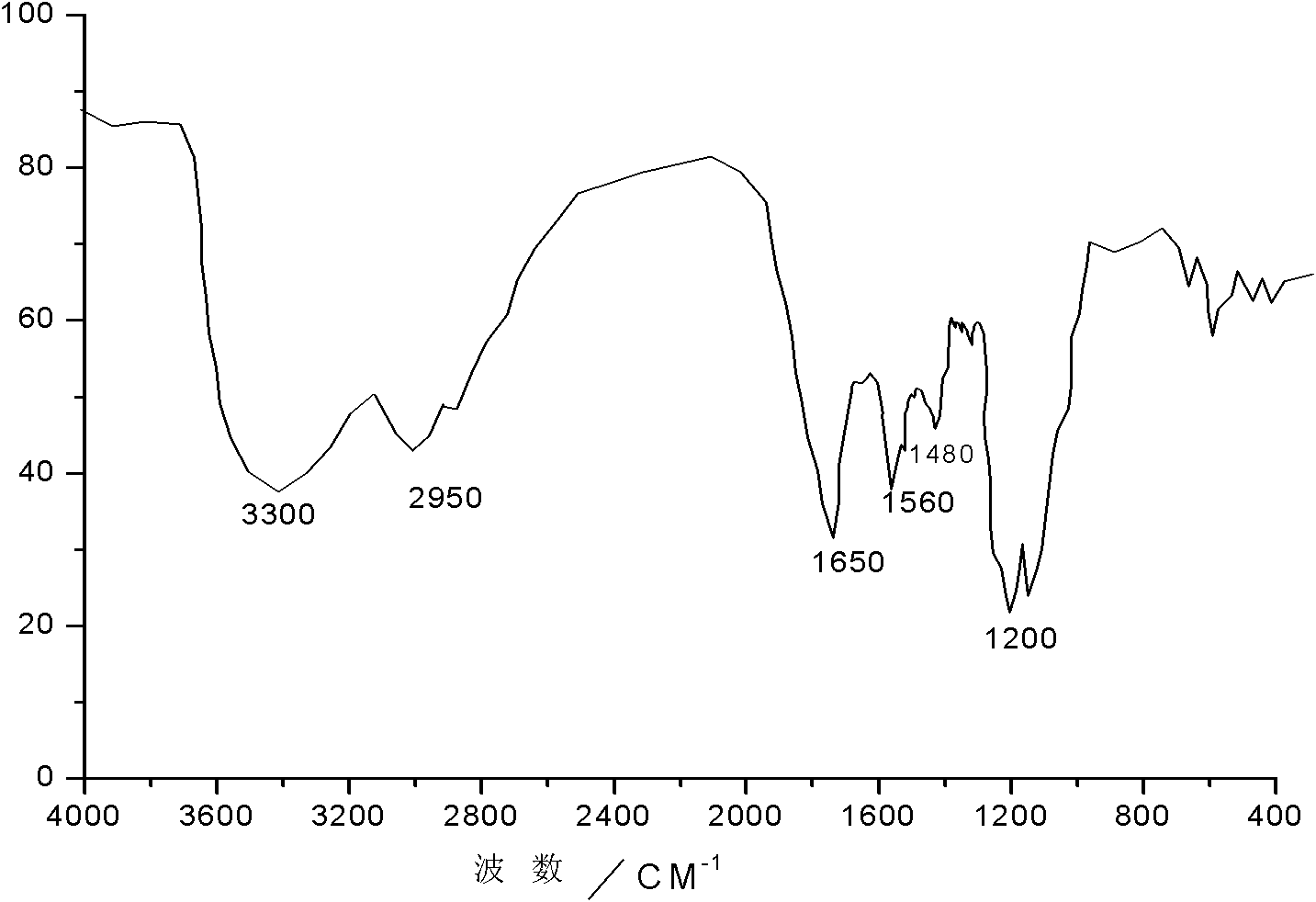
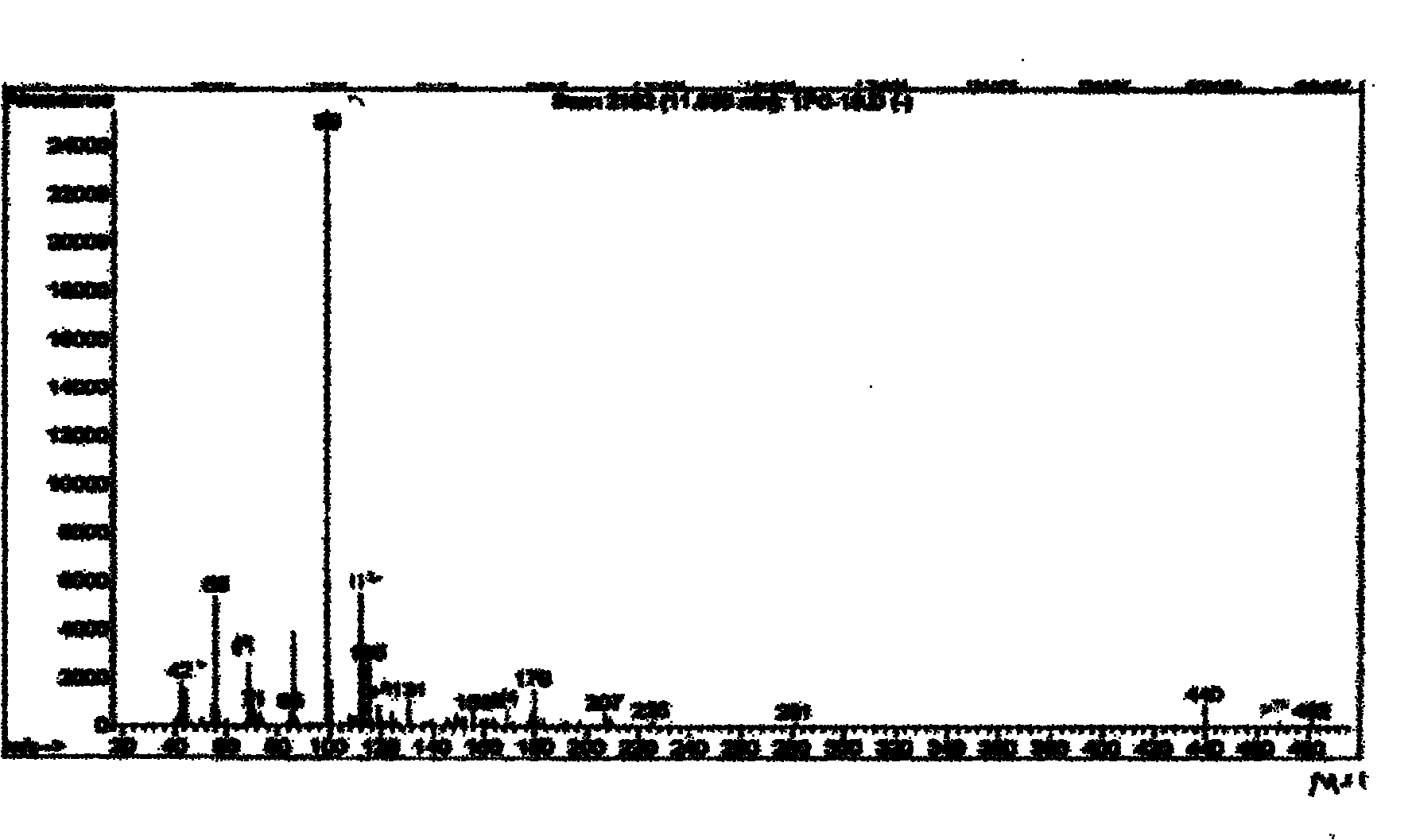
[0030] In a 500ml stainless steel reaction kettle equipped with a thermometer, agitator and pressure gauge, add 207g of perfluorooctanoic acid, heat to 65°C and stir evenly, then add 80g of diethylenetriamine, add phosphotungstic acid / mesoporous alumina yttrium catalyst 1g, seal the reaction kettle. The system was heated to 120°C, reacted for 8 hours under medium speed stirring, and cooled to room temperature within 2 hours without separation, directly added 64g of benzyl chloride and then heated to 100°C, and reacted for 10 hours under low speed stirring to obtain N-ethylamino-2- Perfluoroheptyl imidazoline quaternary ammonium salt. The structural formula of the N-ethylamino-2-perfluoroheptyl imidazoline quaternary ammonium salt prepared in this embodiment is shown in (1) (2), wherein R 1 for CF 3 -(CF 2 ) 6 , R 2 Ethyl N-alkylamino-2-perfluoroalkyl imidazoline quaternary ammonium salt. The infrared spectrum and mass spectrum of the product are as follows figure 1 , 2...
Embodiment 3
[0038] In a 1000L stainless steel reaction kettle with temperature control, agitator and pressure gauge, add 157kg of perfluorocaproic acid, heat to 65-70°C and stir evenly, then add 124kg of diethylenetriamine, and add 500g of zirconate heteropolyacid catalyst , Airtight reactor. Heat the system to 110-120°C, react for 8-10 hours under medium-speed stirring, and then cool down to room temperature within 2 hours without separation, directly add 126kg of dimethyl sulfate and heat up to 100-110°C, and react for 8-10 hours under low-speed stirring 10h, the obtained N-ethylamino-2-perfluorohexyl imidazoline quaternary ammonium salt. The N-ethylamino-2-perfluorohexyl imidazoline quaternary ammonium salt structural formula that the present embodiment makes is shown in (1) (2), wherein R 1 for R 1 for CF 3 -(CF 2 ) 5 , R 2 for R2 Ethyl N-alkylamino-2-perfluoroalkyl imidazoline quaternary ammonium salt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com