Letrozole I-type crystal and preparation method thereof
A letrozole and crystallization technology, applied in the field of letrozole type I crystallization and its preparation, can solve the problems of inconsistent physical and chemical properties of active substances, uneven properties of final products of preparations, difficult preparation molding technology, etc., and it is easier to achieve quality The effect of control, rapid and complete crystallization, simple and practical process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [Example 1] Preparation of 1-[bis(4-cyanophenyl)methyl]-1,2,4-triazole (letrozole) control crystal form
[0044] The crude 1-[bis(4-cyanophenyl)methyl]-1,2,4-triazole compound of the control crystal form can be prepared by the method described in US Patent No. 20,070,100,149: add 20 g of the crude letrozole to 140 ml dichloromethane, and then 500 ml of methanol were added. Stir for 10 min, remove part of the solvent by rotary evaporation at a temperature below 40°C until 105-120ml of mixed solvent remains, add 350ml of water, and stir for 1h. A large amount of white solid formed and was filtered, washed with 140ml of water at the same time, and suction-filtered under reduced pressure at 55-60°C for 4 hours to obtain 15.2g of white crystalline powder with a yield of 76%. Its XRD spectrogram, DSC spectrogram refer to U.S. Patent US 20,070,100,149, see Figure 4 , Figure 5 .
Embodiment 2
[0045] [Example 2] Preparation of 1-[bis(4-cyanophenyl)methyl]-1,2,4-triazole (letrozole) type I crystal
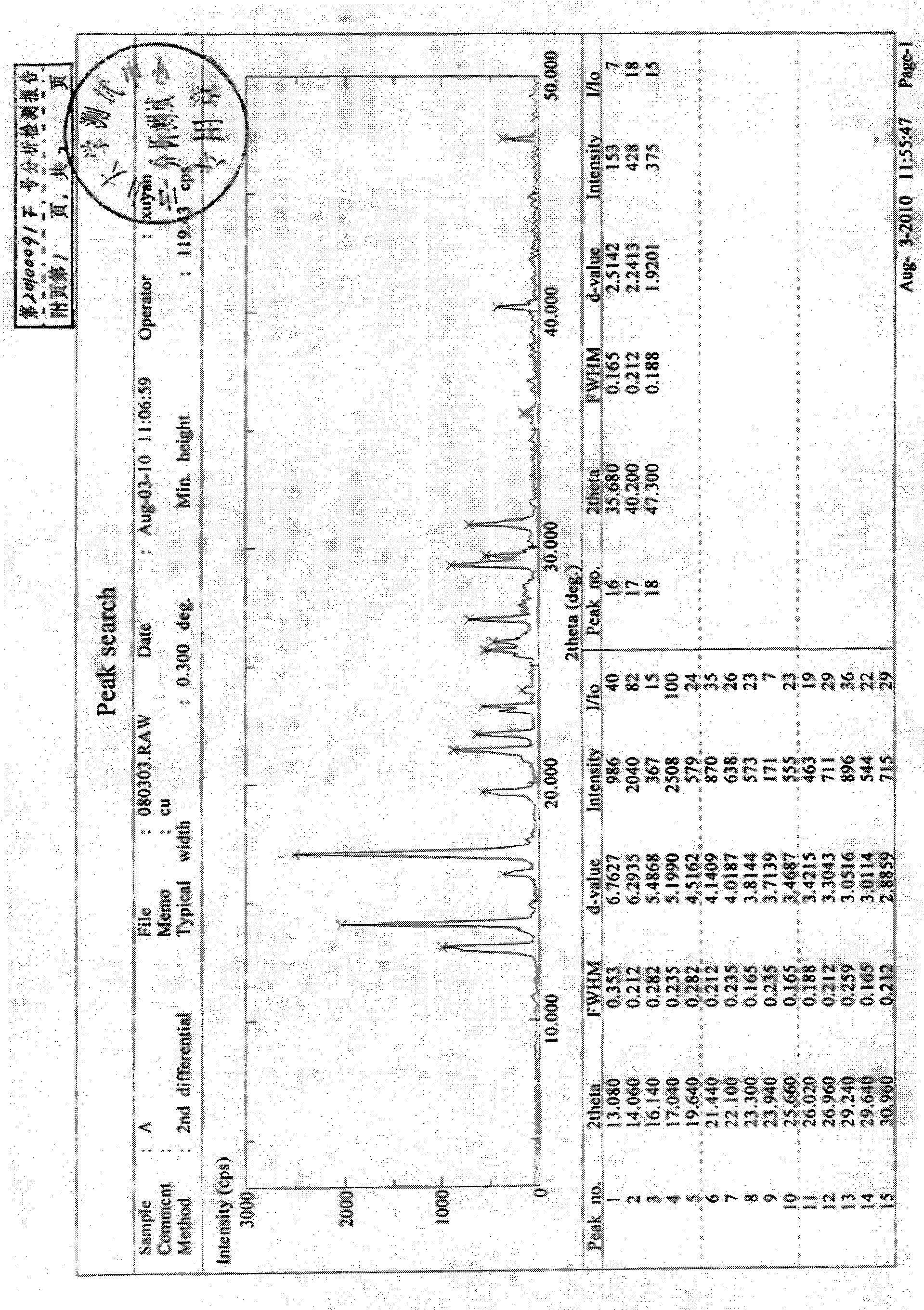
[0046] Add anhydrous potassium carbonate to solvent acetone, shake well, reflux for 4 hours to distill, and put 4ALinde molecular sieves into the collection bottle at the same time to obtain anhydrous acetone solvent. Add 50g of letrozole crude product into about 1.5L of anhydrous acetone solvent, dissolve and filter, and concentrate the filtrate under reduced pressure (0.1Mpa, 50°C), concentrate and dry to constant weight (0.1Mpa, room temperature), and obtain 43g of white crystalline solid , yield 86%.
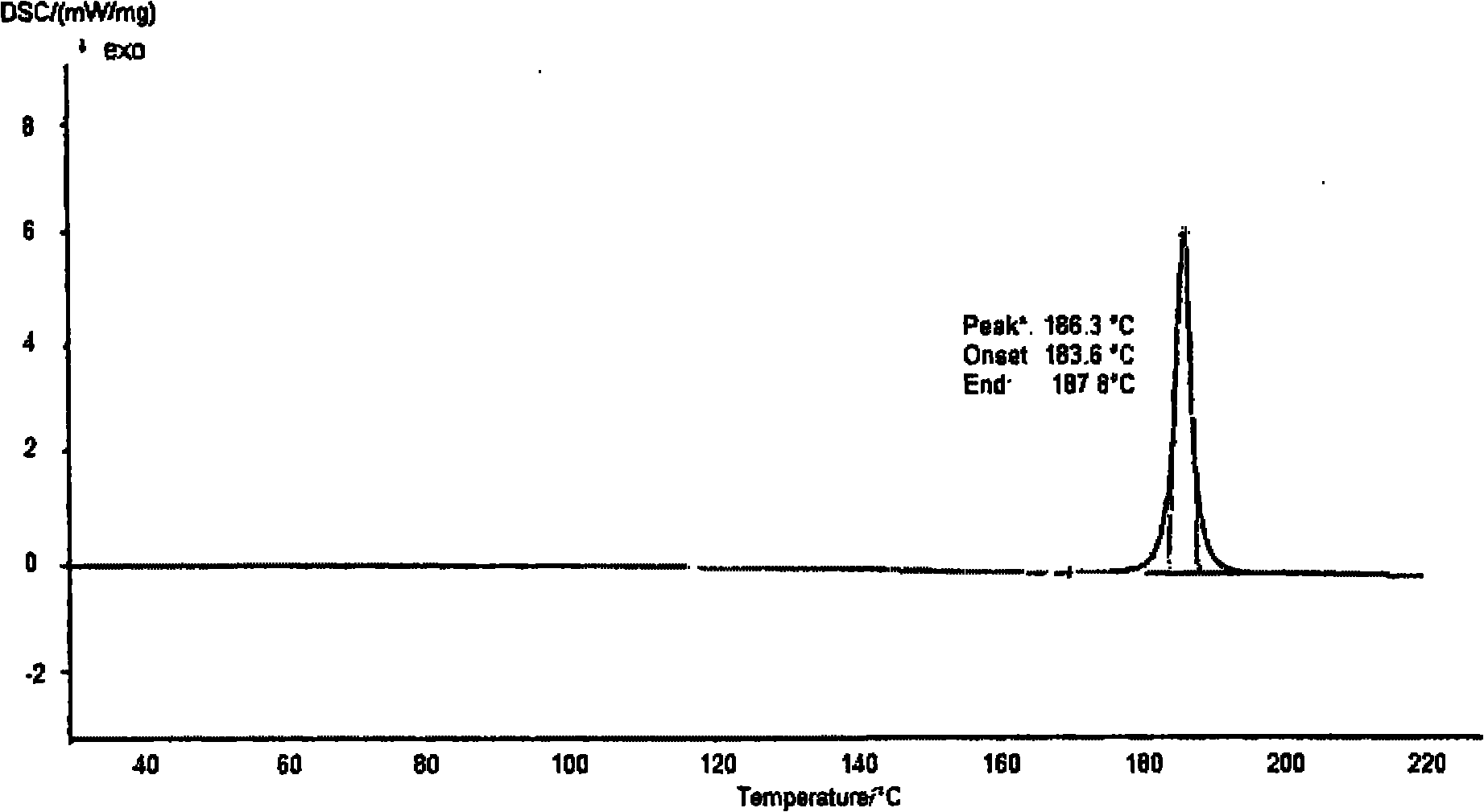
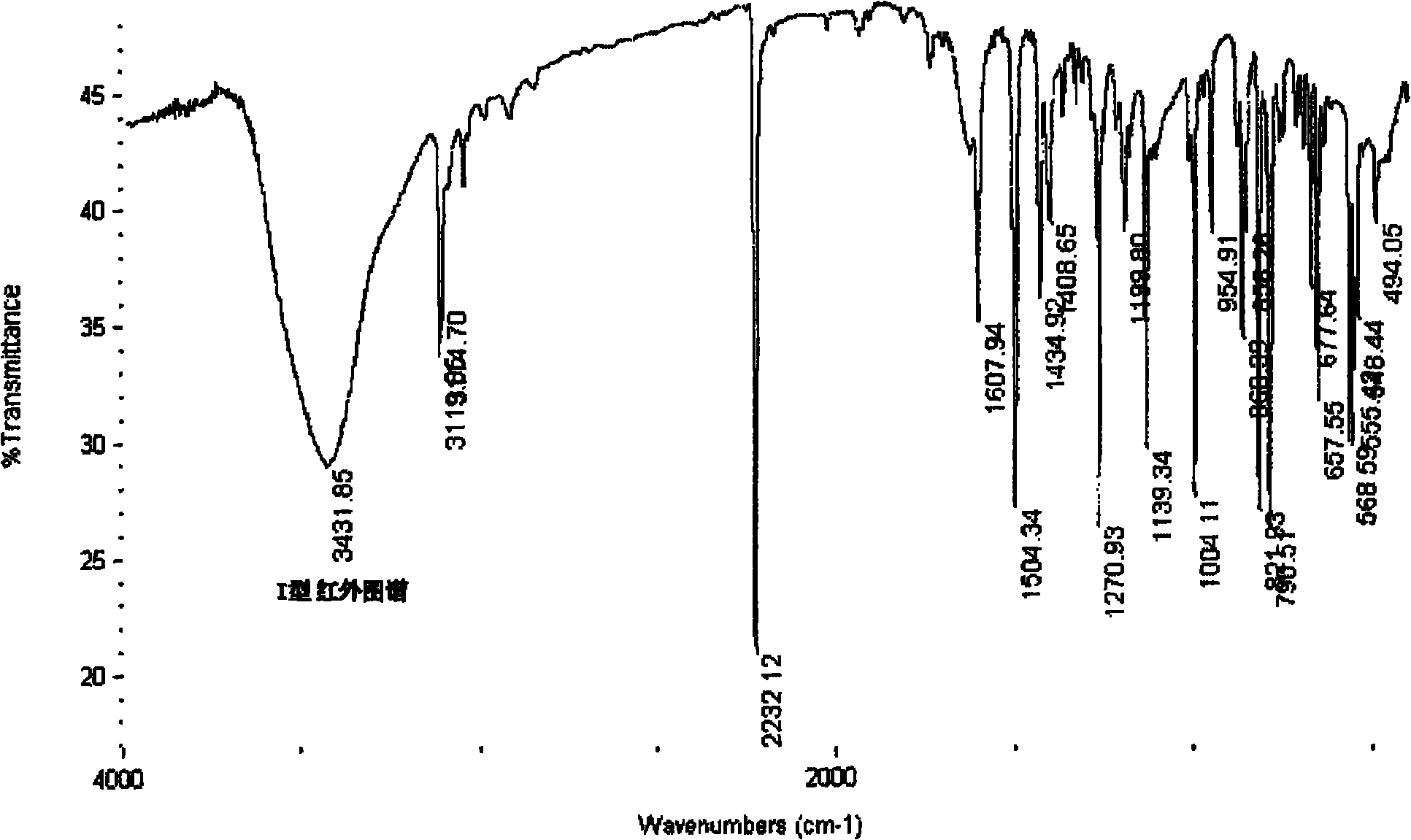
[0047] Its XRD spectrum is as figure 1 As shown, the DSC spectrum is as figure 2 As shown, the infrared spectrum as image 3 shown. The main diffraction peaks of the XRD spectrum are shown in Table 1.
[0048] Table 1
[0049] peak
Embodiment 3
[0050] [Example 3] Preparation of 1-[bis(4-cyanophenyl)methyl]-1,2,4-triazole (letrozole) type I crystal
[0051] Calcium oxide (200g / L) was added into the solvent isopropanol and refluxed for 3 hours, and then distilled, and the distillate was further distilled by adding calcium hydride to obtain anhydrous isopropanol solvent. Add 50g of letrozole crude product into about 1.5L of anhydrous isopropanol solvent, dissolve and filter, concentrate the filtrate under reduced pressure (0.1Mpa, 50°C), concentrate and dry to constant weight (0.1Mpa, room temperature), and obtain 40g of white Crystalline solid, yield 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com