Charge controlling agent and toner using metal compound of cyclic phenol sulfide
A metal compound, charge control agent technology, applied in the development agent, electrographics, optics, etc., can solve the problems of large variation of toner charging characteristics, easy variation of copy image quality, lack of clarity of copied image, etc. Good reproducibility of fine lines, excellent environmental stability, excellent effect of dispersibility and stability of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] The present invention will be described below in conjunction with examples, but these examples do not limit the present invention in any way. In the examples, "parts" all mean "parts by mass".
[0170] In a 1L four-necked flask equipped with a stirrer, a cooling tube, a thermometer and a gas introduction tube, 120.2g (0.8mol) of 4-tert-butylphenol, 51.3g (1.6mol) of monomer sulfur and 16.0g (0.4mol) of sodium hydroxide were added ), add 360.5g of diphenyl ether to it, keep it at 130°C while stirring in a nitrogen stream, and carry out the reaction for 1 hour while removing the water and hydrogen sulfide produced by the reaction, and then heat up to 170°C to remove the reaction The generated water and hydrogen sulfide were reacted for 1 hour, and then the temperature was increased to 230°C, and the reaction was performed for 18 hours while removing the water and hydrogen sulfide generated by the reaction. The reaction mixture was cooled to room temperature, and after addin...
Embodiment 2
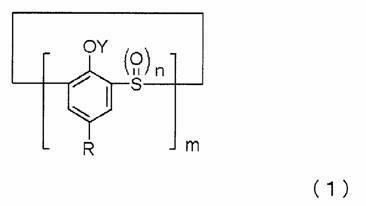
[0176] Add 8.65 g (0.012 mol) of the above-mentioned refined product obtained in Example 1 into a 500 ml four-necked flask, add 300 ml of the above-mentioned boric acid buffer solution (pH 8.5-8.6), and heat to 50°C. 2.99g (0.012mol) of cobalt acetate tetrahydrate suspended in 100ml of the above-mentioned boric acid buffer (pH8.5~8.6) was added dropwise over 10 minutes. After dripping the dark pink cobalt acetate, the reaction liquid changed to light blue. The pH after the dripping was completed was 7.5. After stirring for 4 hours while heating to 50°C, it was cooled to room temperature. The reaction product was collected by filtration under reduced pressure, washed 3 times with 50 ml of water, and dried under reduced pressure at 120°C to obtain a brownish yellow powder in the general formula (1) where R is tert-butyl, m is 4, and n is 0, Y is 9.29g of the compound of the present invention with cobalt.
Embodiment 3
[0178] In a 500ml four-necked flask equipped with a stirrer, a cooling tube, and a thermometer, 56.2g of cyclic phenol sulfide in which R is tert-butyl, m is 4, n is 0, and Y are all hydrogen atoms in the general formula (1) is added ( 0.078 mol), 224.8 g (4 times wt / wt) of acetic acid, 5.15 g (0.0156 mol) of sodium tungstate dihydrate, 5.31 g (0.039 mol) of sodium acetate trihydrate, and the temperature was raised to 60°C while stirring . While stirring, 121.2 g (1.248 mol) of a 35% hydrogen peroxide aqueous solution was added dropwise to the flask over about 1.5 hours. After the dropwise addition, the mixture was stirred at 60°C for 15 hours and then at 70°C for 15 hours. Next, 15.8 g (0.156 mol) of 36% hydrochloric acid was added dropwise at 80°C while stirring, and then stirred at 80°C for 1 hour. After cooling to room temperature, a white solid precipitated. The white solid was collected by filtration under reduced pressure, washed 3 times with 80 ml of water, and dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com