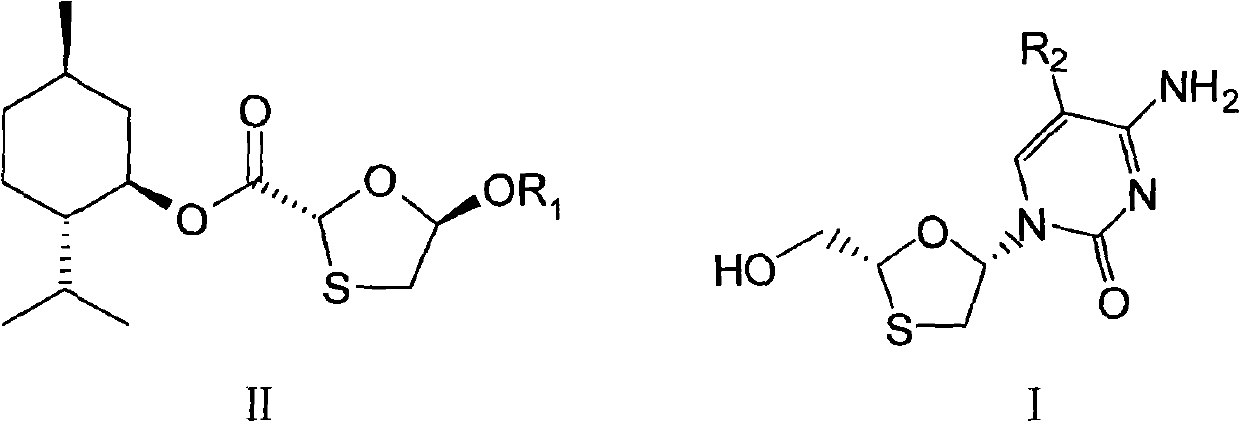
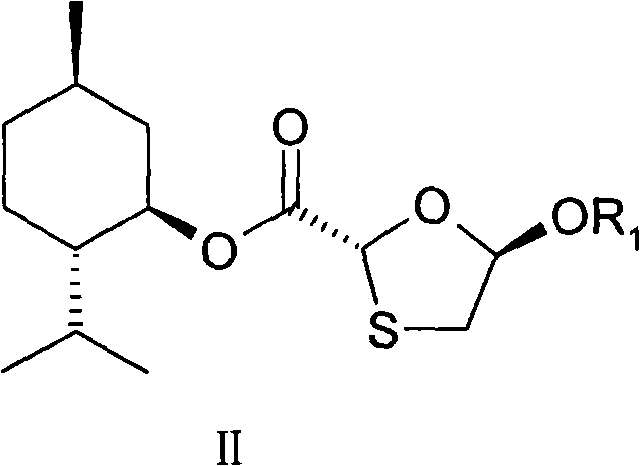
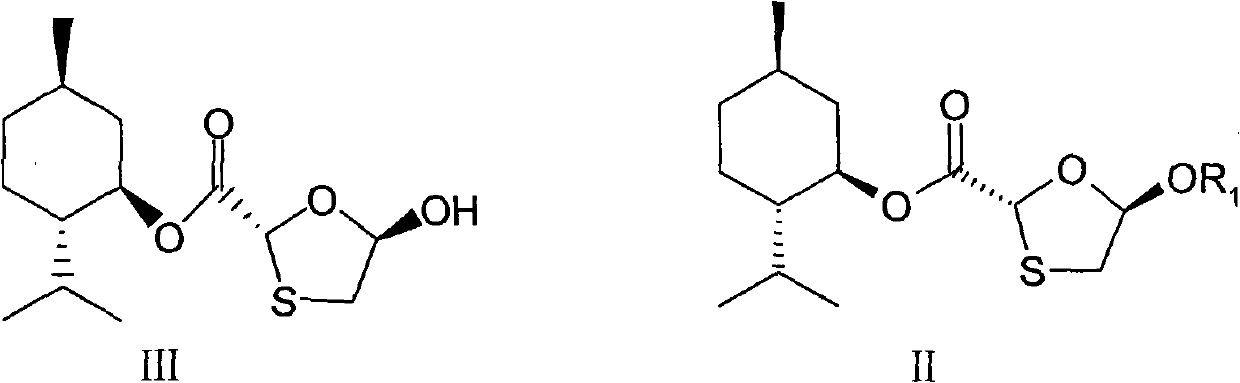
High optical purity nucleoside intermediates and preparation method thereof
A technology of optical purity and nucleosides, applied in organic chemistry and other directions, can solve the problems of low HPLC purity, inoperability, low optical purity, etc., and achieve the effect of reducing production costs, avoiding complicated operations, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (5R)-Acetoxy-1,3-oxathiolane-(2R)-carboxylic acid-[(1′R, 2′S, 5′R)-5′-methyl-2′- Preparation of (1-methylethyl)cyclohexyl] ester (IIA)
[0080]
[0081] 5-Hydroxy-1,3-oxathiolane-2-carboxylic acid [(1'R, 2'S, 5'R)-5'-methyl-2'-(1-methylethyl Base) cyclohexyl] ester (III) 200g into a 2L three-necked flask, add 4-dimethylaminopyridine (10.16g) and tetrahydrofuran (600ml), stir to dissolve, cool to -30 ~ -20 ° C, in 2 ~ 3 hours Add 99ml of acetic anhydride dropwise, continue the reaction for 45-60min after the dropwise addition, then slowly add 10% sodium carbonate aqueous solution dropwise to adjust the pH to 7, transfer it to a separatory funnel, let it stand for layers, and wash the water layer with 100ml×2 tetrahydrofuran Extracted twice, collected the organic layer, dried overnight with anhydrous magnesium sulfate, filtered, washed the filter cake with a small amount of tetrahydrofuran, concentrated the filtrate under reduced pressure at 60°C, and recrystallized w...
Embodiment 2
[0100] (5R)-propionyloxy-1,3-oxathiolane-(2R)-carboxylic acid-[(1′R, 2′S, 5′R)-5′-methyl-2′ Preparation of -(1-methylethyl)cyclohexyl]ester (IIB)
[0101]
[0102] 5-Hydroxy-1,3-oxathiolane-2-carboxylic acid [(1'R, 2'S, 5'R)-5'-methyl-2'-(1-methylethyl Base) cyclohexyl] ester (III) 200g into a 2L three-necked flask, add 25.4g of 4-dimethylaminopyridine and 1000ml of dichloromethane, stir to dissolve, cool to -20~-10℃ in an ice-salt bath, slowly add 110ml dropwise Propionic anhydride, add dropwise within 1.5 hours, continue to react for 1 hour, then slowly add 10% sodium carbonate aqueous solution dropwise to adjust the pH to 7, transfer to a separatory funnel, let stand to separate layers, and extract the water layer with 100ml×2 dichloromethane Twice, collect the organic layer, dry over anhydrous magnesium sulfate, filter, wash the filter cake with a small amount of dichloromethane, concentrate the filtrate under reduced pressure at 50°C, and recrystallize with 600ml of 9...
Embodiment 3-10
[0126] Embodiment 3-10 (wherein embodiment 6-10 is comparative example)
[0127] According to the above operation, the reaction is carried out under different reaction temperature conditions, and the other conditions are kept unchanged to obtain the nucleoside intermediate compound of formula II.
[0128]
[0129]
[0130] From the above comparison experiments, the reaction temperature has a great influence on the purity of the compound of formula II, and the optical purity of the compound of formula II obtained when the reaction temperature is controlled at -30~-5°C is obviously higher than the temperature in other reaction ranges, reaching high optical purity. purity while maintaining or significantly increasing yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com