Industrialized semisynthesis of medicine-vincamine for treating cerebral ischemia
A technology of vincamine and nitrogen oxides, applied in the direction of organic chemistry, can solve the problems of low chemical atom economy, high production equipment requirements, high cost, etc., and achieve the goal of green production process, simple equipment, large economic and social benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
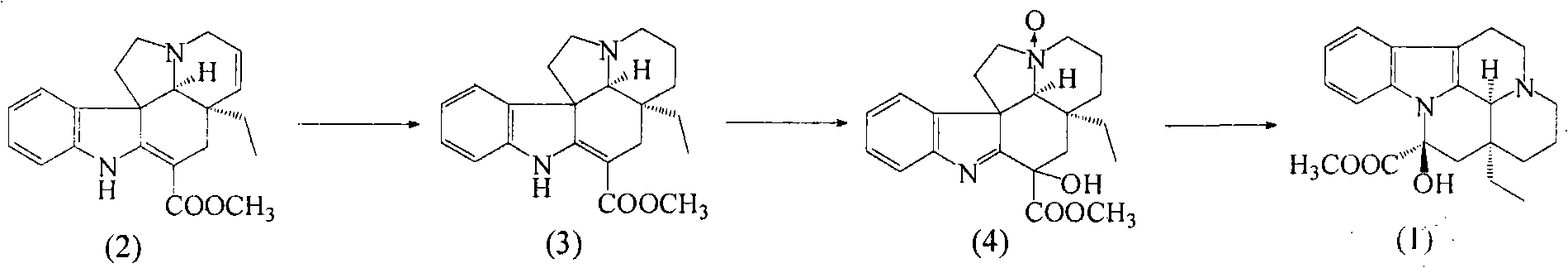
[0019] Embodiment 1 Synthesis of Changchun Fermin (3)
[0020] Weigh 5.0 kg of tarponin hydrochloride (2) into a 100 L stainless steel reaction kettle, add 50 L of 75% ethanol, and stir to dissolve at room temperature. Then 250g of Raney nickel was added, hydrogen was introduced immediately, stirred after 0.5h, and the hydrogen pressure was controlled to 1.1atm, and the reaction was carried out for 8h. After the reaction was completed, nitrogen gas was introduced for 10 minutes, the catalyst was removed by filtration, and the crude product of vincaurmin was obtained by concentration. Crystallization with ethyl acetate-ethanol gave 4.8 kg of vincaurmin hydrochloride with a purity greater than 99.0%, with a yield of 96%.
Embodiment 2
[0021] Example 2 Synthesis of 1,2-dehydro-16-methoxycarbonyl-16-hydroxyl-vinfofermin nitrogen oxide (4)
[0022] Take 5.0 kg of vinchun phermin hydrochloride and place it in a 100 L stainless steel reaction kettle, add 50 L of 1,3-dioxane, stir and dissolve at room temperature. Filled with nitrogen protection, protected from light and reacted at room temperature for about 16 hours. TLC detects the progress of the reaction until the intermediate (3) disappears substantially, and the intermediate (4) is generated. Add 5% NaHCO to the reaction solution 3 The solution was free of bubbles, and the excess peroxyacid and the generated acid were removed. Separate the organic phase, then extract twice with 20L dichloroethane, combine the organic phases, dry over 500 g of anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 1,2-dehydro-16-methoxycarbonyl-16-hydroxyl - The crude product of vinchun fermin nitrogen oxide (structural formula 4), which is an oily sub...
Embodiment 3
[0023] The synthesis of embodiment 3 vincamine (1)
[0024] Take 5.0kg of 1,2-dehydro-16-methoxycarbonyl-16-hydroxy-vinfofermin nitrogen oxide and place it in a 100L stainless steel reaction kettle, add 60L of trichloroacetic acid, control the temperature at about 0°C and stir to dissolve, then add Triphenylphosphine 3.0kg, reflux reaction for about 3h. TLC detects the progress of the reaction until the intermediate (4) disappears substantially. Cool the reaction solution, add an equal amount of ice water, and wash away triphenylphosphine oxide with dichloroethane. The aqueous phase was then adjusted to pH 8-9 with 1% NaOH, extracted twice with dichloromethane, the combined organic phases were concentrated to 20 L volume, and decolorized by adding activated carbon for 30 min. The organic phase was obtained by filtration, dried overnight with 500 g of anhydrous sodium sulfate, and a certain amount of methanol was added to the mother liquor to crystallize to obtain the crude p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com