Battery
A battery and electrolyte technology, applied in the field of electrochemical energy storage, can solve the problems of limiting battery development, poor cycle performance, vanadium toxicity, etc., and achieve the effect of easy manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
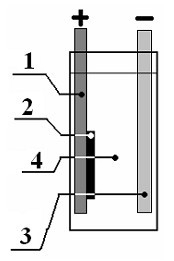
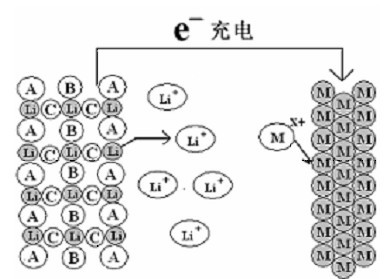
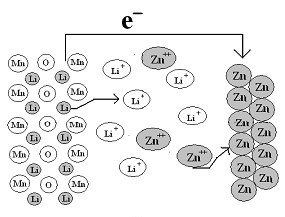
[0046] Take LiMn 2 o 4 It is the positive electrode active material, according to the ratio of positive electrode active material 88wt%: conductive carbon black 8wt%: binder PTFE (polytetrafluoroethylene) 4wt%, mix evenly, cut into a disc with a diameter of 12mm and a thickness of 0.1-0.2mm, and press On the graphite current collector, a positive electrode is made. The negative electrode is zinc metal with a width of 15mm and a thickness of 1mm, which also serves as a current collector. The distance between positive and negative electrodes is 5mm, and the diaphragm is filter paper. The electrolyte is a mixed aqueous solution of lithium chloride and zinc chloride containing 1mol / L lithium ions and 5mol / L zinc ions. The cycle test is carried out under the condition of a voltage range of 1.3-2.05V and a charge and discharge current of 0.5C. attached figure 1 The basic structure of the battery is shown. Its first charge and discharge curve is shown in the attached image 3...
Embodiment 2
[0048] The battery is manufactured in the same manner as in Example 1, except that LiCoO 2 As a positive electrode active material, the cycle operating voltage range is 1.3-1.95V. The battery cycle performance test results are shown in Table 1.
Embodiment 3
[0050] The battery is manufactured in the same manner as in Example 1, except that LiFePO 4 As a positive electrode active material, the cycle operating voltage range is 0.8-1.6V. The battery cycle performance test results are shown in Table 1 and attached Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com