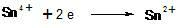Method for restoring stannic chloride
A technology of tin tetrachloride and tin protochloride, which is applied in the direction of tin protochloride and tin halide, can solve the problems of secondary pollution, and achieve the effects of low cost, reasonable synthesis process and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1. A method for reducing tin tetrachloride, the method uses electricity as a reducing agent; its specific steps are as follows: add the use of tin protochloride to reduce diazo The dilute hydrochloric acid solution of tin tetrachloride produced by the salt is stirred and energized to 15A; the temperature is raised slowly and reacted at 70°C; after detecting that there is no tetravalent tin in the solution, the reaction ends, and the dilute hydrochloric acid of tin protochloride is obtained solution.
Embodiment 2
[0012] Example 2. A method for reducing tin tetrachloride, the method uses electricity as a reducing agent; its specific steps are as follows: in an electrolytic cell equipped with a tail gas treatment system, stirring, a nickel electrode as the cathode, and a metal electrode as the ruthenium iridium titanium as the anode, add Utilize the dilute hydrochloric acid solution of tin tetrachloride produced by the reduction of diazonium salt by tin protochloride, start stirring, and turn on electricity to 22A; slowly heat up and react at 65°C; after detecting that there is no tetravalent tin in the solution, the reaction ends, that is A dilute hydrochloric acid solution of stannous chloride was obtained.
Embodiment 3
[0013] Example 3. A method for reducing tin tetrachloride, the method uses electricity as a reducing agent; its specific steps are as follows: in an electrolytic cell equipped with a tail gas treatment system, stirring, a nickel electrode as the cathode, and a metal electrode of iridium, tantalum and titanium as the anode, add Utilize the dilute hydrochloric acid solution of tin tetrachloride produced by the reduction of diazonium salt by tin protochloride, start stirring, and turn on electricity to 18A; slowly heat up and react at 75°C; after detecting that there is no tetravalent tin in the solution, the reaction ends, that is A dilute hydrochloric acid solution of stannous chloride was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com