Method for extracting aluminum oxide from pulverized flue ash
A technology of fly ash and alumina, applied in alumina/aluminum hydroxide and other directions, can solve the problems of difficult reaction, high equipment investment, large material volume, etc., and achieves low corrosion resistance requirements, low equipment corrosion, and residues. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
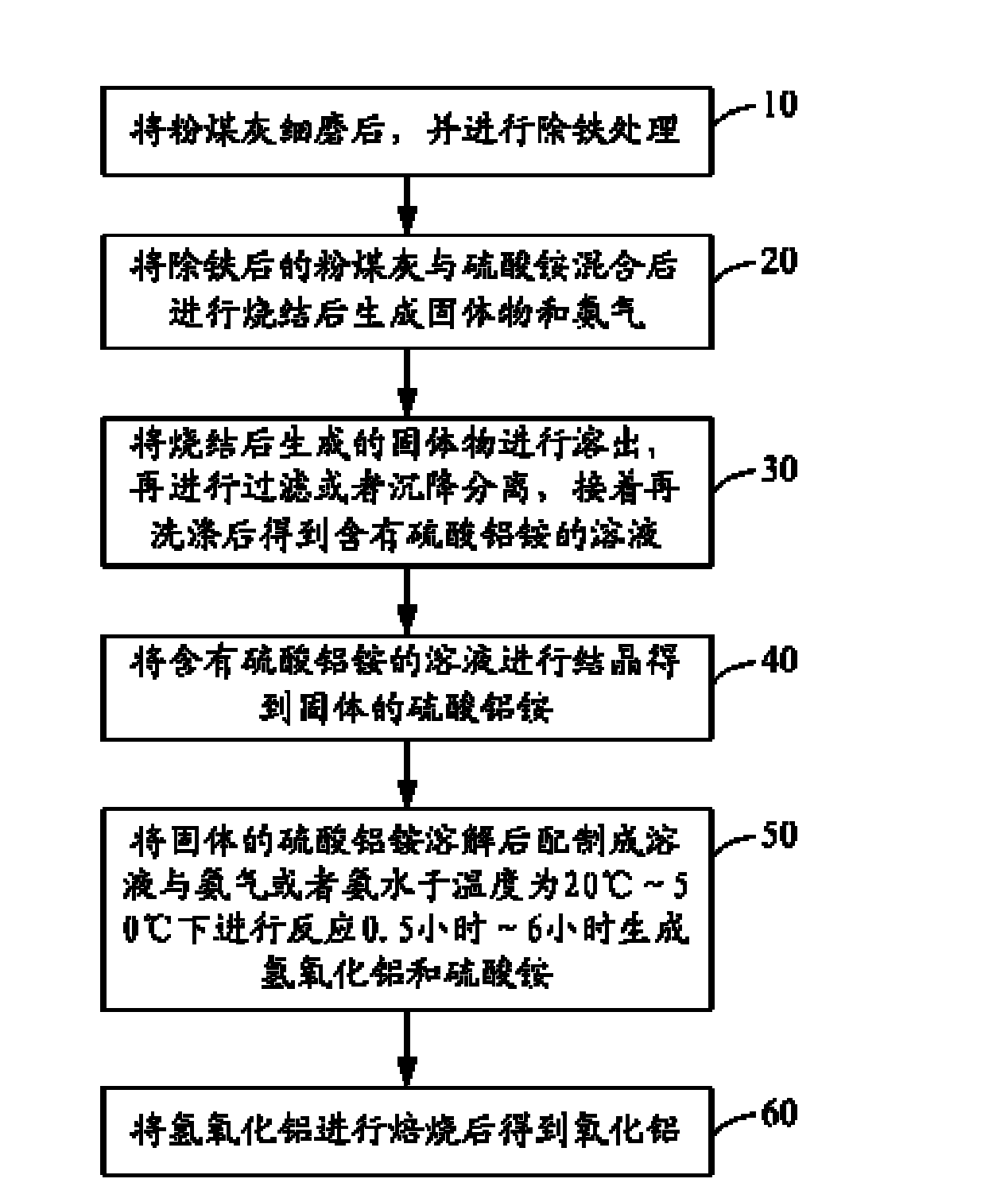
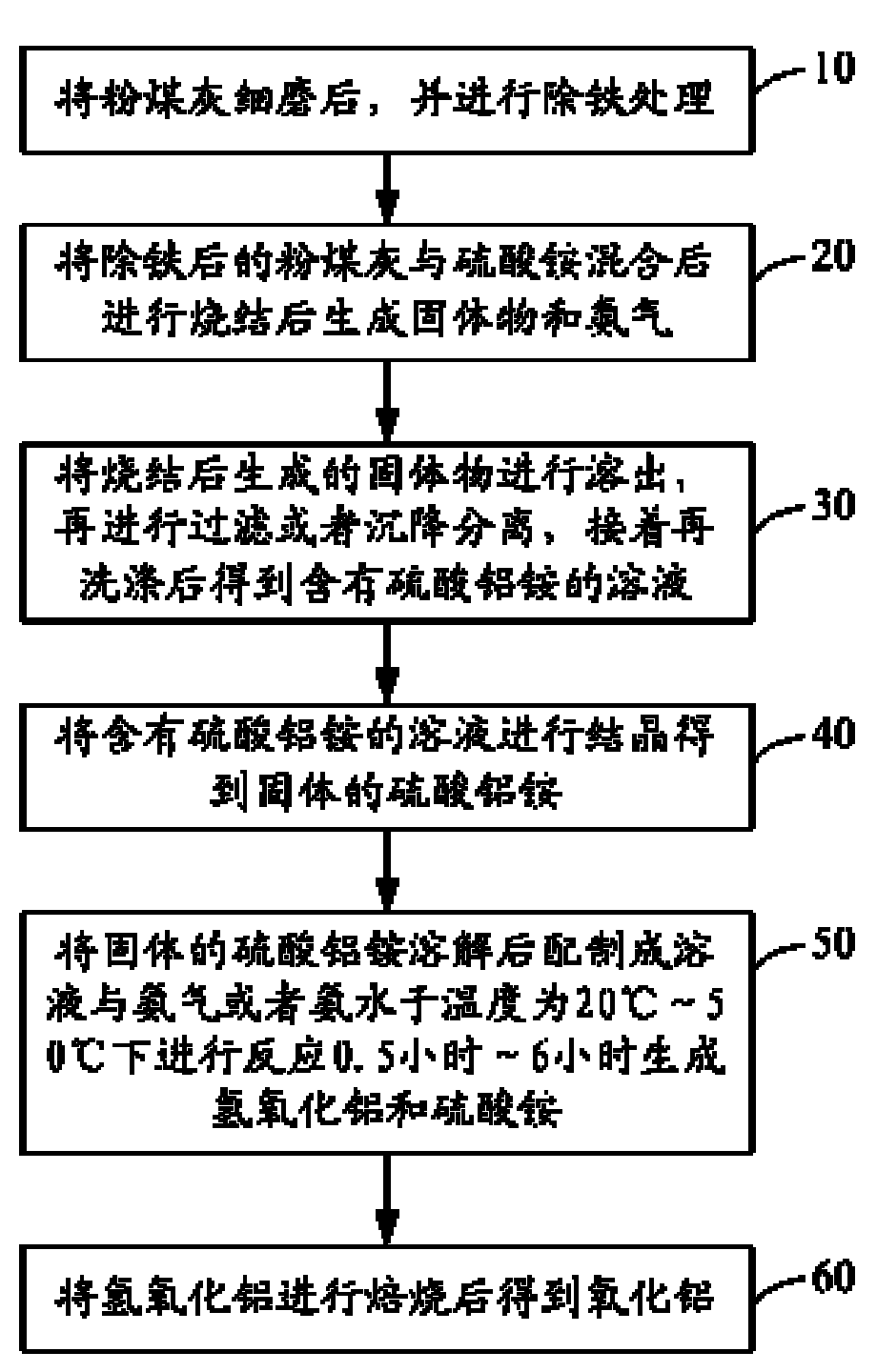
[0047] Grind the fly ash containing 46% of alumina and 40% of silica to 1000 mesh, weigh ammonium sulfate and fly ash according to the mass ratio of ammonium sulfate / alumina in fly ash to 10.0, and sinter the sintering temperature The temperature is 720°C, the sintering time is 3.5 hours, the sintered clinker is dissolved with water at 98°C under normal pressure, the dissolution rate of alumina in the obtained ammonium aluminum sulfate solution is 87%, and the ammonium aluminum sulfate solution is removed after iron and other impurities , the aluminum ammonium sulfate solution was cooled to 5°C for crystallization for 3 hours, and then the obtained aluminum ammonium sulfate crystals were dissolved to prepare a 0.2mol / L aluminum ammonium sulfate solution, and ammonia gas was introduced to decompose it. The reaction temperature was 35°C, and the reaction After 3 hours, the aluminum hydroxide solid whose particle size is less than 1.5% of the total number of particles that can lea...
Embodiment 2
[0049] Grind the fly ash containing 42% alumina and 40% silica to 200 meshes, weigh ammonium sulfate and fly ash according to the mass ratio of ammonium sulfate / alumina in fly ash to 8.5, and sinter the sintering temperature The temperature is 500°C, the sintering time is 3.5 hours, and the sintered clinker is dissolved with water at 98°C under normal pressure, and the dissolution rate of aluminum oxide in the obtained ammonium aluminum sulfate solution is 89%. After the ammonium sulfate solution is removed from iron and other impurities, Cool the aluminum ammonium sulfate solution to 10°C for crystallization for 5 hours, then dissolve the obtained aluminum ammonium sulfate crystals to prepare a 0.25mol / L aluminum ammonium sulfate solution, then react with ammonia water with a concentration of 25%, and control the reaction temperature to 45 ℃, the reaction time is 2 hours, and the particle size is that the number of particles that can leak through the mesh of 325 mesh accounts ...
Embodiment 3
[0051] Grind the fly ash containing 40% alumina and 43% silica to 800 meshes, weigh ammonium sulfate and fly ash according to the mass ratio of ammonium sulfate / alumina in fly ash to 7.2, and sinter the sintering temperature The temperature is 320°C, the sintering time is 4.0 hours, the sintered clinker is dissolved with water at 98°C under normal pressure, and the dissolution rate of aluminum oxide in the obtained ammonium aluminum sulfate solution is 92%. After the ammonium sulfate solution is removed from impurities such as iron, Cool the aluminum ammonium sulfate solution to 8°C for crystallization for 4 hours, then dissolve the obtained aluminum ammonium sulfate crystals to prepare a 0.19 mol / L aluminum ammonium sulfate solution, and feed ammonia gas to react. The reaction temperature is controlled at 60°C, and the reaction time is After 3 hours, the aluminum hydroxide solid whose particle size is less than 1.8% of the total number of particles that can leak through the 32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com