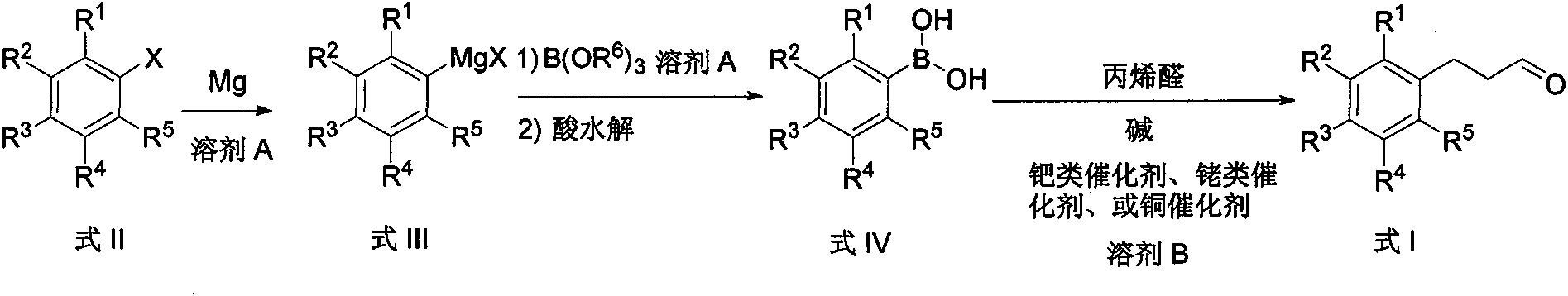
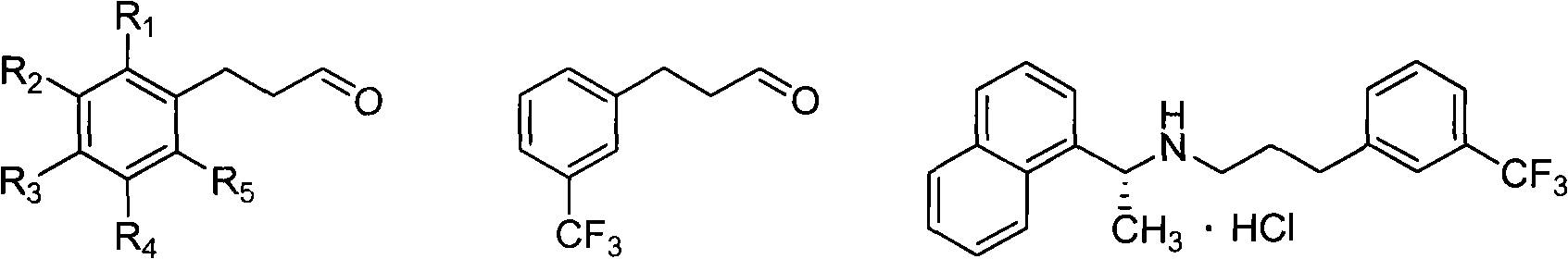
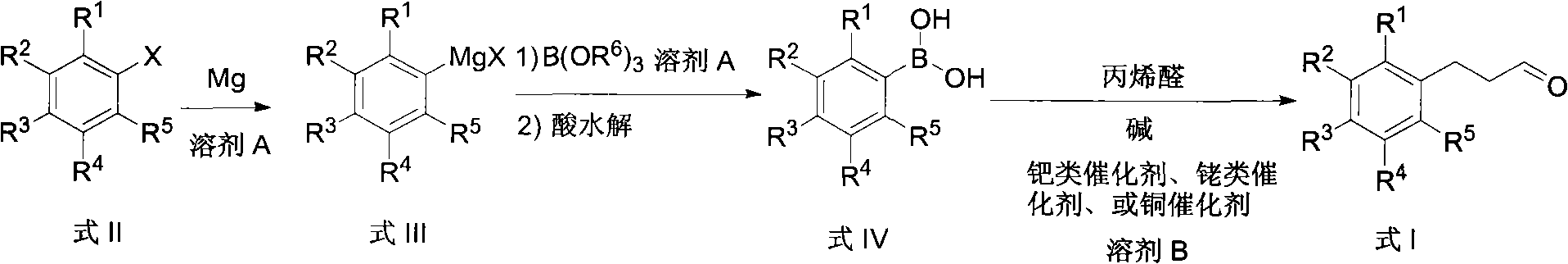
Preparation method of arylpropylaldehyde derivatives
A technology for aryl propionaldehyde and derivatives, which is applied in the field of preparation of aryl propionaldehyde derivatives, can solve the problems of harsh reaction conditions, high production equipment requirements, and poor operability, and achieve easy industrial production and low production equipment requirements , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of m-trifluoromethyl phenylpropanal
[0045] Preparation of m-trifluoromethylphenylboronic acid
[0046] Under nitrogen, m-trifluoromethylbromobenzene (2.3 g, 0.01 mol), magnesium (2.7 g, 0.11 mol), 10 mL of ether and 50 mg of iodine were added to a flask equipped with a stirrer, condenser, and thermometer, The color of iodine induced by heating disappeared. Then m-trifluoromethylbromobenzene (23 g, 0.1 mol) and 60 ml of diethyl ether were dropped into the flask at 30° C., keeping a slight boil. After dropping, the mixture was stirred and reacted at reflux for 2 hours. After the reaction, cool to room temperature, add trimethyl borate (114.6 g, 1.1 mol) cooled to -78°C and 20 ml of diethyl ether, and stir for 12 hours. Warming up to room temperature, pouring into 1 mol / L hydrochloric acid aqueous solution for acid hydrolysis. After the acid hydrolysis was completed, 100 ml of ethyl acetate was added to extract and separate the organic l...
Embodiment 2
[0049] Embodiment 2: the preparation of p-tert-butylphenylpropionaldehyde
[0050] Preparation of p-tert-butylphenylboronic acid
[0051] Under nitrogen, add p-tert-butylbromobenzene (2.1 g, 0.01 mol), magnesium (2.9 g, 0.12 mol), 10 mL THF and 50 mg iodine to a flask equipped with a stirrer, condenser and thermometer, and heat cause. Then, p-tert-butylbromobenzene (21.3 g, 0.1 mol) and 60 ml of tetrahydrofuran were dropped into the flask at 40° C., keeping slight boiling. After dropping, reflux and stir the reaction for 1 hour. After the reaction, cool to room temperature, and add tributyl borate (24.4 g, 0.12 mol) and 30 ml tetrahydrofuran cooled to -78°C. The reaction was stirred for 12 hours. Warming up to room temperature, pouring into 1 mol / L sulfuric acid aqueous solution for acid hydrolysis. After the acid hydrolysis was completed, 100 ml of ethyl acetate was added, the organic layer was separated, and the aqueous layer was extracted twice with 100 ml of ethyl ace...
Embodiment 3
[0054] Embodiment 3: the preparation of phenylpropionaldehyde
[0055] Preparation of phenylboronic acid
[0056] Under nitrogen, bromobenzene (1.6 g, 0.01 mol), magnesium (3.2 g, 0.13 mol), 10 ml of tetrahydrofuran and 50 mg of iodine were added to a flask equipped with a stirrer, condenser and thermometer, and heated to initiate. Then bromobenzene (15.7 g, 0.1 mol) and 60 ml of tetrahydrofuran were dropped into the flask at 40° C., keeping a slight boil. After dropping, the reaction was stirred at 25°C for 3 hours. After completion of the reaction, cool to room temperature, add tributyl borate (24.4 g, 0.12 mol) cooled to -78°C and 30 ml THF, and stir for 72 hours. Warming up to room temperature, pouring into 1 mol / L sulfuric acid aqueous solution for acid hydrolysis. After the acid hydrolysis was completed, 100 ml of ethyl acetate was added, the organic layer was separated, and the aqueous layer was extracted twice with 100 ml of ethyl acetate. The organic layers were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com