Method for preparing aryl propanal derivatives
A technology for aryl propionaldehyde and derivatives, which is applied in the field of preparation of aryl propionaldehyde derivatives, can solve the problems of harsh reaction conditions, high production equipment requirements, high pressure on safety and environmental protection, etc., and achieves easy industrial production and production equipment requirements. Low, three-waste emissions are small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
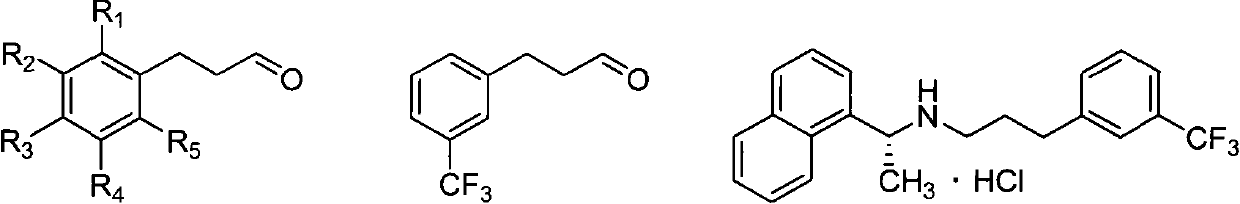
[0043] Embodiment 1: the preparation of m-trifluoromethyl phenylpropanal
[0044] Preparation of 1-(3-ethoxyallyl)-3-(trifluoromethyl)benzene
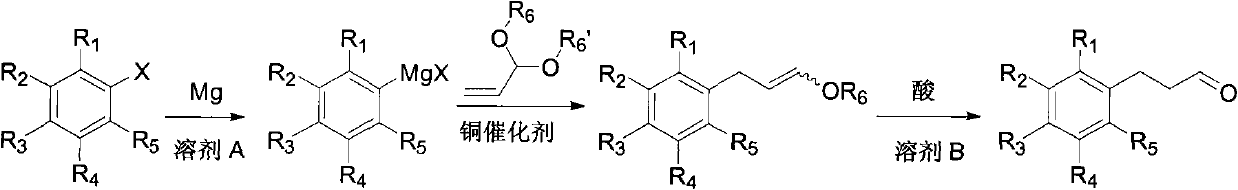
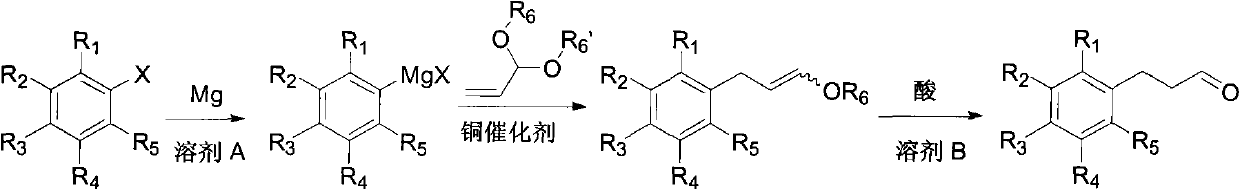
[0045] Under nitrogen, m-trifluoromethylbromobenzene (23 g, 0.1 mol), magnesium (29 g, 1.2 mol), 60 ml of ether and 50 mg of iodine were added to the reaction flask, heated to initiate the reaction, and then m-trifluoromethyl Bromobenzene (230 g, 1 mole) and 360 ml of diethyl ether were dropped into the reaction flask and kept boiling slightly. After dropping, the mixture was stirred and reacted at reflux for 2 hours. After the reaction was completed, it was cooled to room temperature, and acrolein diethyl acetal (14.3 g, 0.11 mol) and cuprous iodide (1.9 g, 0.01 mol) were added. The reaction was stirred at reflux for 12 hours. After the reaction, the pH value was adjusted to 7 with saturated ammonium chloride aqueous solution at 0°C. Diethyl ether was distilled off, 50 ml of ethyl acetate was added, the organic layer was separated...
Embodiment 2
[0057] Embodiment 2: the preparation of p-tert-butylphenylpropionaldehyde
[0058] Preparation of 1-tert-butyl-4-(3-ethoxyallyl)benzene
[0059] Under nitrogen, p-tert-butylbromobenzene (2.1 g, 0.01 mole), magnesium (4 g, 0.17 mole), 10 milliliters of tetrahydrofuran and 50 mg of iodine were added to the reaction flask, heated to initiate the reaction, and p-tert-butylbromobenzene (21.3 g, 0.1 mol) and 60 ml of tetrahydrofuran were dropped into the reaction flask. After dropping, the reaction was stirred at -20°C for 72 hours. After the reaction was completed, it was cooled to room temperature, acrolein diethyl acetal (13 g, 0.1 mol) and cuprous bromide (0.7 g, 0.005 mol) were added, and the reaction was stirred at -20°C for 72 hours. After the reaction, the pH value was adjusted to 7 with saturated ammonium chloride aqueous solution at 0°C. The tetrahydrofuran was distilled off, 50 ml of ethyl acetate was added, the organic layer was separated, and the aqueous layer was ex...
Embodiment 3
[0062] Embodiment 3: the preparation of phenylpropionaldehyde
[0063] Preparation of 1-(3-methoxyallyl)benzene
[0064] Under nitrogen, bromobenzene (1.6 g, 0.01 mole), magnesium (3.2 g, 0.13 mole), 10 milliliters of tetrahydrofuran and 50 mg of iodine were added to the reaction flask, and the reaction was initiated by heating, and bromobenzene (15.7 g, 0.1 mole) and 60 milliliters of tetrahydrofuran was dripped into the reaction flask and kept boiling slightly. After dropping, the reaction was stirred at 25°C for 3 hours. Cool to room temperature after the reaction, add acrolein dimethyl acetal (10.2 g, 0.1 mol) and cuprous chloride (9.9 g, 0.1 mol), and react under reflux for 12 hours. After the reaction, the pH value was adjusted to 8 with saturated ammonium chloride aqueous solution at 0°C. The tetrahydrofuran was distilled off, 50 ml of ethyl acetate was added, the organic layer was separated, and the aqueous layer was extracted twice with 50 ml of ethyl acetate. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com