Method for synthetizing basic orange II artificial antigen
A technology of artificial antigen and synthesis method, applied in animal/human protein, color/spectral property measurement, albumin peptide, etc., to meet the needs of research and the effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] (1) Preparation of artificial hapten
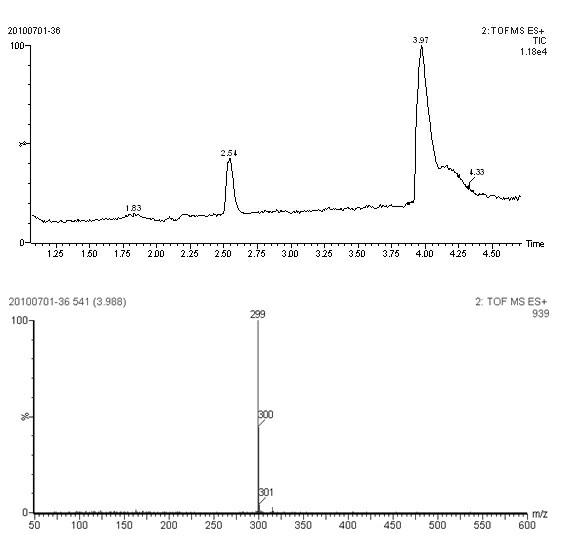
[0025] Solution A: Dissolve 50mg of p-aminobenzenebutyric acid in 2mL of absolute ethanol, adjust the pH to 1~2, add sodium nitrite until the starch potassium iodide test paper turns blue, and react for 1 hour.
[0026] Liquid B: Dissolve 35 mg of m-phenylenediamine in 2 mL of absolute ethanol to prepare Liquid B.
[0027] Add solution A to solution B dropwise, keep the pH at 8-9 during the reaction, and react for 3 hours to prepare the artificial hapten.
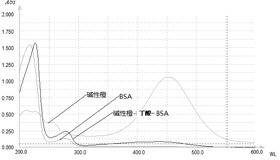
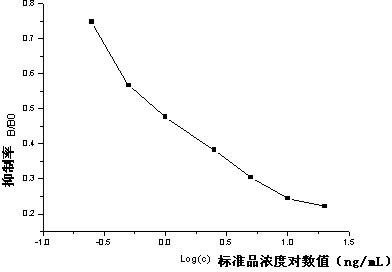
[0028] (2) Preparation of complete antigen
[0029] Solution C: Dissolve 8 mg of artificial hapten, 6 mg of EDC, and 4 mg of NHS in 2 mL of PBS (0.01M), and stir magnetically for 3 h.
[0030] Solution D: Dissolve 20mg BSA in 0.05M pH 9.6 carbonate buffer, store at 4°C until use.
[0031] Add solution C dropwise to solution D in an ice bath, and then incubate on ice at 4°C for 3 hours to obtain the artificial antigen mixture.
[0032] Dialysis bag pre-treatment: Take a 10cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com