Compound azo salicyl aldehyde semicarbazone mercaptothiodiazole and preparation and application thereof
A technology of azosalicylaldehyde, amino mercaptothiadiazole and nitroazosalicylaldehyde, which is used in analysis by chemical reaction of materials, organic chemistry, material analysis by observing the effect on chemical indicators, etc. It can solve the problem of less acetate ions, and achieve the effect of strong complexing ability and high detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of azosalicylaldehyde aminomercaptothiadiazole
[0039] (1) Synthesis of 2-amino-5-mercapto-1,3,4-thiadiazole
[0040] Using N,N-dimethylformamide as the solvent, mix thiosemicarbazide and carbon disulfide at a molar ratio of 1:1.7, stir and reflux at 82°C for 5-8 hours, cool, evaporate the solvent under reduced pressure, and add a concentration of 2.1mol ·L -1 sodium hydroxide solution (the molar weight of sodium hydroxide is 1.35 times that of thiosemicarbazide), and then use 4 mol·L -1 Concentrated hydrochloric acid acidified to pH 1~2 to obtain milky white precipitated crude product, suction filtered, dried, and recrystallized from a mixture of ethanol and water (volume ratio of ethanol to water 2:1) to obtain 2-amino-5-mercapto -1,3,4-Thiadiazole.
[0041] (2) Synthesis of the p-nitroazo salicylaldehyde
[0042] Using distilled water as a solvent, mix p-nitroaniline with sulfuric acid with a mass fraction of 30-60% (the molar mass ...
Embodiment 2
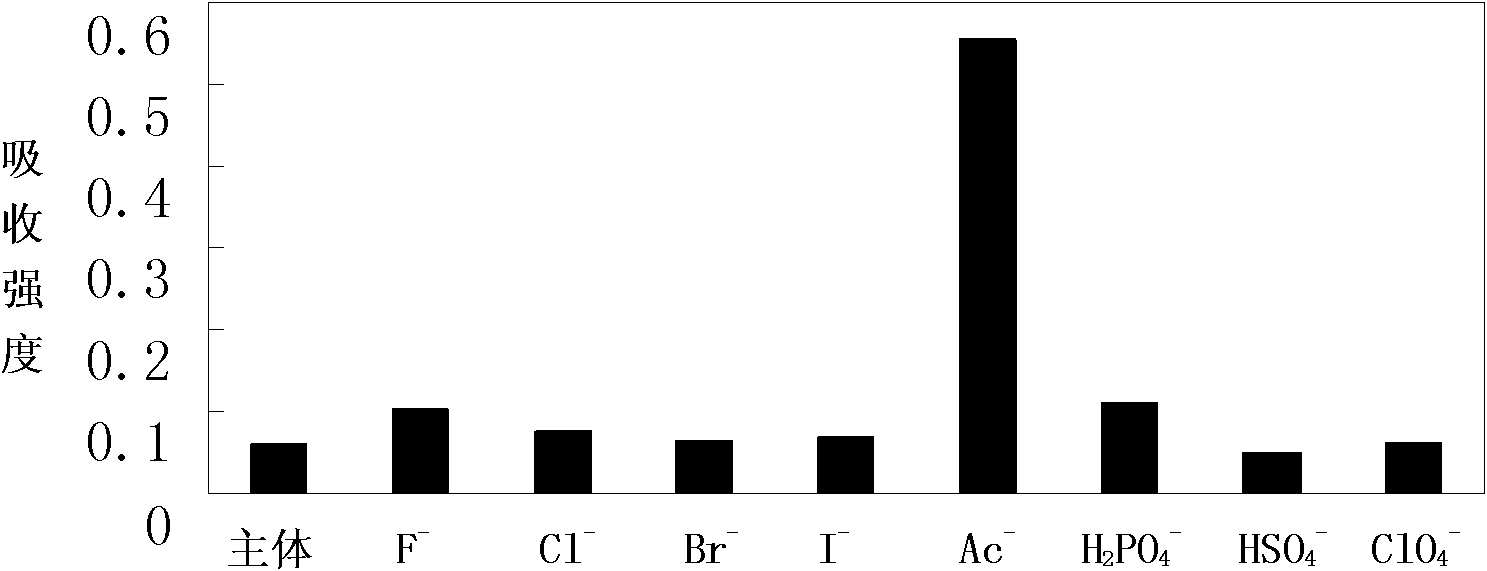
[0048] Embodiment 2, colorimetric identification Ac -
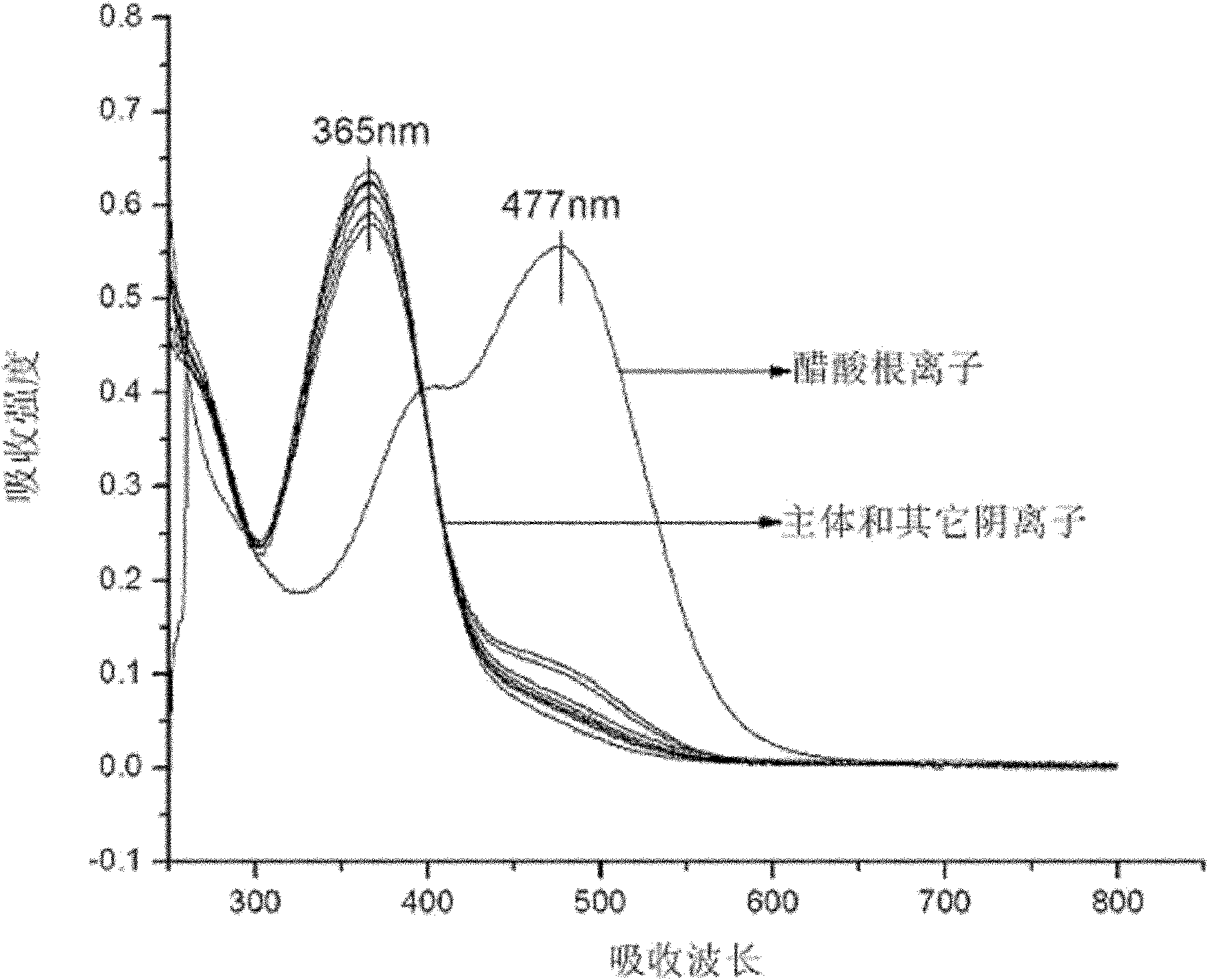
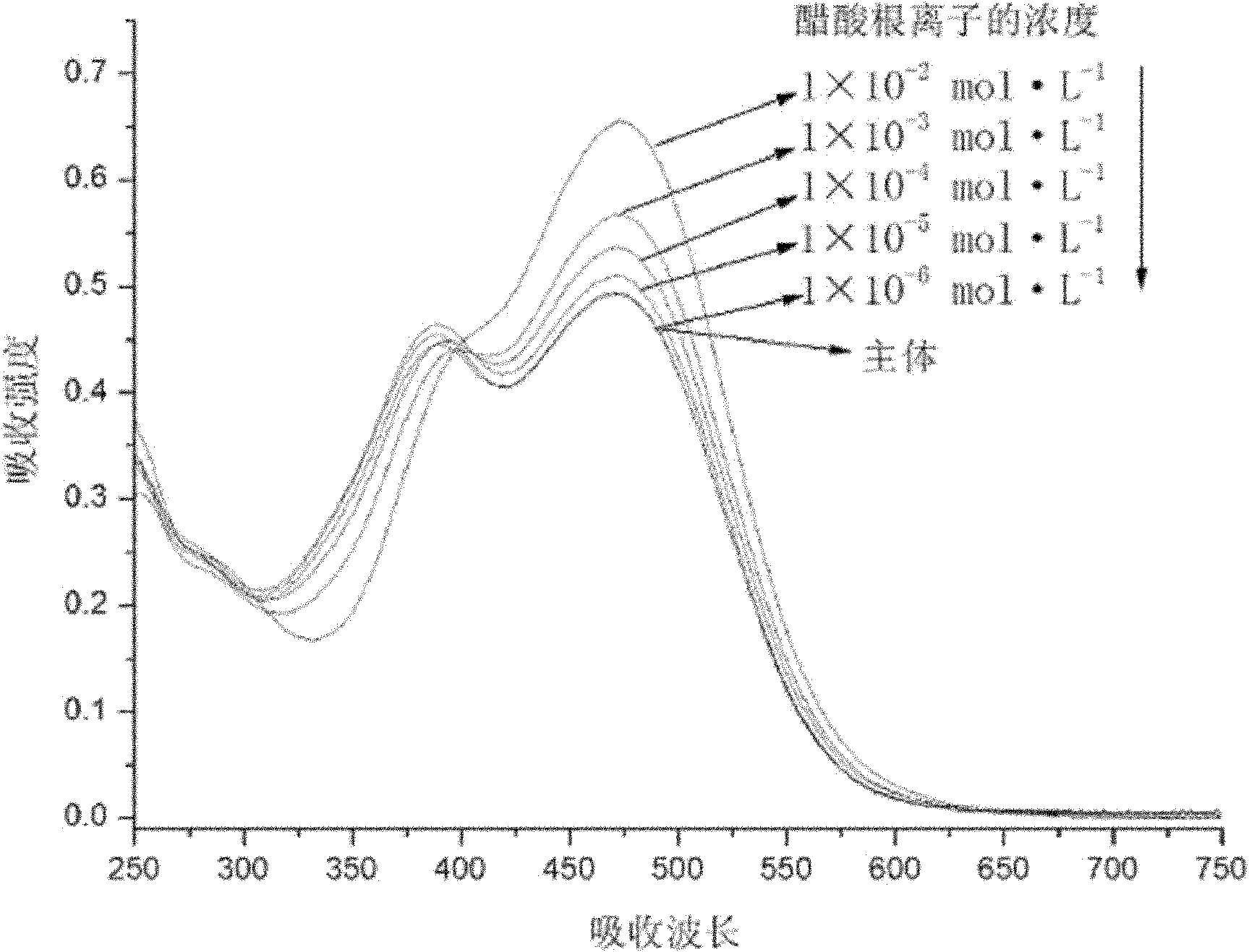
[0049] Pipette 0.5 mL receptor DMSO solution (2×10 -4 mol L -1 ) in a series of 10 mL colorimetric tubes, then pipette 2.5 mL of triple distilled water into the above colorimetric tubes with receptors added, and then add F - , Cl - 、 Br - , I - 、 Ac - , H 2 PO 4 - , HSO 4 - and ClO 4 - DMSO solution of tetrabutylammonium salt (1 x 10 -2 mol L -1 ) 0.5ml. Dilute to 5mL with DMSO, at this time the receptor concentration is 2×10 -5 mol L -1 , the anion concentration is 50 times that of the acceptor, and it is observed immediately after mixing evenly: when in the acceptor's DMSO / H 2 When the DMSO solution of the above-mentioned anions is added to the O solution, Ac - The addition of DMSO / H to the acceptor 2 The color of the O solution changed from light yellow to orange-yellow. In its corresponding UV spectrum, Ac - The addition of the acceptor completely disappears the absorption peak at 365nm, and a s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com