Method for synthesizing 5,7-dihydroxy flavone
A technology of dihydroxyflavone and a synthesis method, which is applied in the field of fine chemical drug synthesis, can solve the problems of not being developed into industrialized production, harsh equipment requirements, and high cost, and achieve the effects of shortening production time, low production cost, and low environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
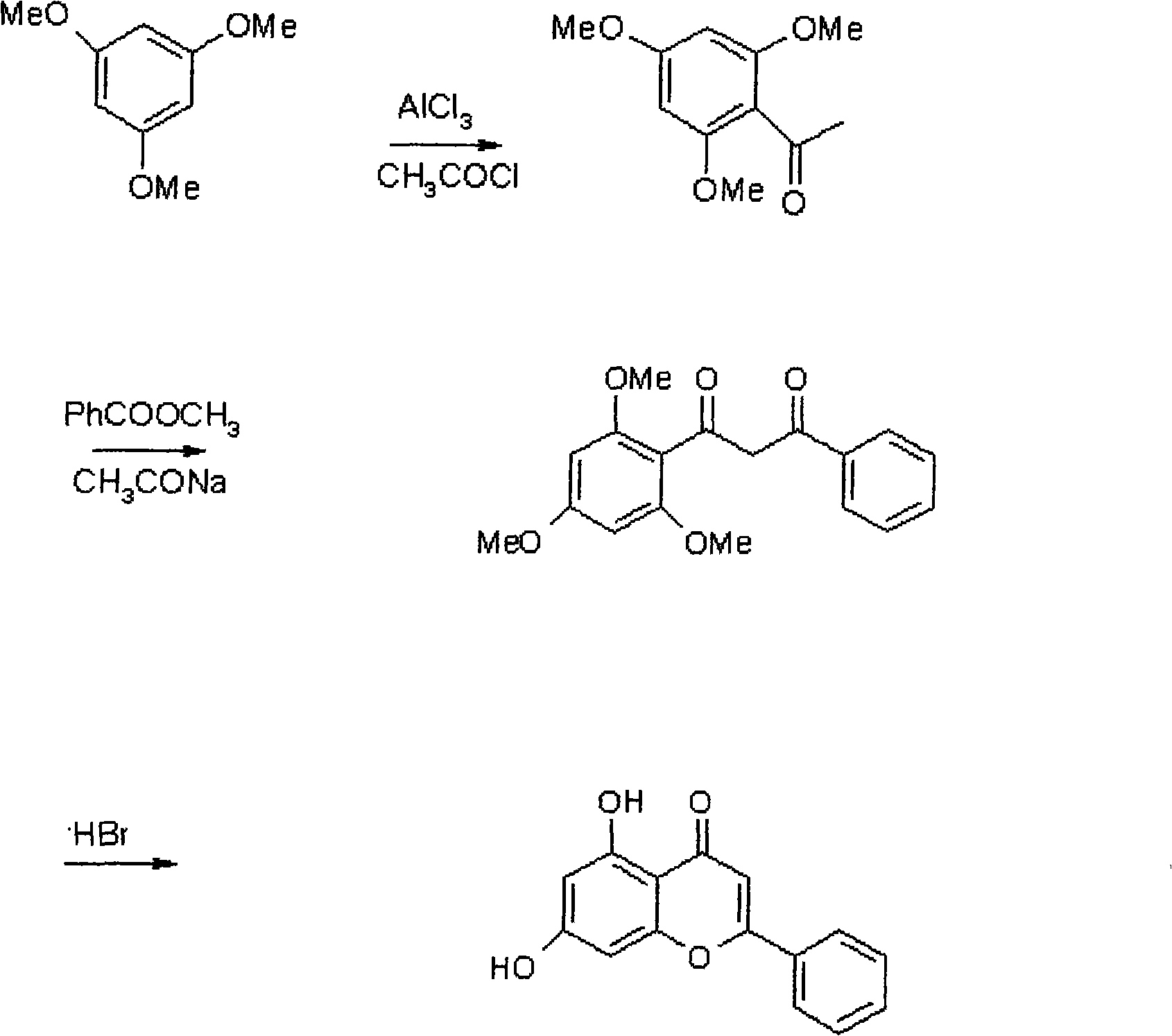
[0033] Step 1: In a 1000ml three-necked reaction flask, add 168g of 1,3,5-trimethoxybenzene, 132g of aluminum trichloride, and 900ml of dichloroethane in sequence, and stir to dissolve. The temperature was controlled in an ice bath at 5°C, and 86 g of acetyl chloride was added dropwise, and the addition was completed within 20 minutes. After the addition, the temperature was raised to room temperature 25°C. Reacted for about 3.5 hours, poured the reaction solution into 2000ml of 5% hydrochloric acid ice solution, stirred for 30 minutes, statically separated and took the organic phase, washed with water until neutral, concentrated under reduced pressure to obtain the residue, and cooled to obtain intermediate 1, 199.5 g. Yield 95%.
[0034] Step 2: Add 13.5g of sodium methoxide powder and 67.5ml of methanol to a 500ml reaction bottle, and heat to reflux for 0.5h. Recover and distill methanol to obtain solid sodium methoxide. Then drop into intermediate 1,42g, methyl benzoat...
Embodiment 2
[0037] Step 1: In a 1000ml three-necked reaction flask, add 168g of 1,3,5-trimethoxybenzene, 158g of aluminum trichloride, and 800ml of dichloroethane in sequence, and stir to dissolve. The temperature was controlled in an ice bath at 5°C, and 93.6 g of acetyl chloride was added dropwise, and the addition was completed within 20 minutes. After the addition, the temperature was raised to room temperature 25°C. After reacting for about 3 hours, pour the reaction solution into 2000ml of 5% hydrochloric acid ice solution, stir for 30 minutes, and separate the layers to get the organic phase, wash with water until neutral, concentrate under reduced pressure to obtain the residue, and cool to obtain Intermediate 1, 182.7g . Yield 87%
[0038]The second step: in the 500ml reaction bottle, add sodium methoxide powder 18.9g, intermediate 142g, methyl benzoate 33g, toluene 350ml, stir, and connect the fractionation column on the reaction device. Heating started, and the temperature o...
Embodiment 3
[0041] Step 1: In a 1000ml three-necked reaction flask, add 168g of 1,3,5-trimethoxybenzene, 146g of aluminum trichloride, and 900ml of dichloromethane in sequence, and stir to dissolve. The temperature was controlled in an ice bath at 5°C, and 82 g of acetyl chloride was added dropwise, and the addition was completed within 20 minutes. After the addition, the temperature was raised to room temperature 25°C. After reacting for about 5 hours, pour the reaction solution into 2000ml of 5% hydrochloric acid ice solution, stir for 30 minutes, and separate the layers to get the organic phase, wash with water until neutral, concentrate under reduced pressure to obtain the residue, and cool to obtain Intermediate 1, 178.5g . Yield 87%.
[0042] Step 2: Add 21.6g of sodium methoxide powder and 108ml of methanol to a 500ml reaction bottle, and heat to reflux for 0.5h. Recover and distill methanol to obtain solid sodium methoxide. Then drop into intermediate 1,42g, methyl benzoate 33...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com