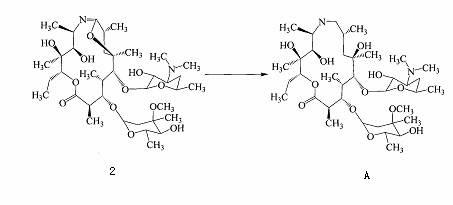
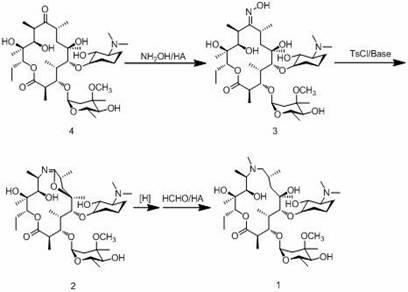
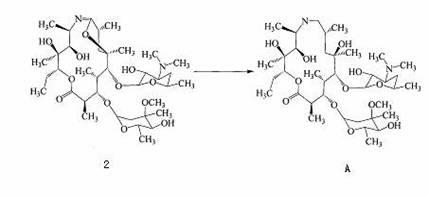
Preparation method of azithromycin intermediate
A technology for azithromycin and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield of azithromycin, easy hydrolysis of boronate intermediates, etc., and achieve the effects of facilitating industrial production, reducing acid degradation products, and reducing preparation costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 erythromycin A6, the preparation of 9-imine ether
[0030] Add 135g of erythromycin A9-oxime and 60g of alkaline agent sodium bicarbonate into a 1000ml reaction bottle, add 540ml of water, lower the temperature below 5°C, add dropwise acetone solution (50ml) of 30ml of methanesulfonyl chloride, finish dripping in 20min, and keep warm For the reaction, use 20% sodium hydroxide solution to adjust the pH value to 10-11, stir for 1 hour, filter with suction, wash with water to obtain the product, dry it, about 127.8g, the yield is 95%, and the HPLC purity content is 95% after testing .
Embodiment 2
[0031] Embodiment 2 erythromycin A6, the preparation of 9-imine ether
[0032] Add 135g of erythromycin A9-oxime and 60g of alkaline agent sodium bicarbonate into a 1000ml reaction bottle, add 540ml of water, lower the temperature below 5°C, add dropwise acetone solution (50ml) of 50ml of p-toluenesulfonyl chloride, and finish dripping in 20 minutes , heat preservation reaction, adjust the pH value to 10-11 with 20% sodium hydroxide solution, stir for 1h, filter with suction, wash with water, get the product, dry it, about 125.8g, the yield is 94%, and the purity content after testing HPLC is 97%.
Embodiment 3
[0033] Example 3 Preparation of 9-deoxy-9a-aza-9a-erythromycin A
[0034] Add 100ml of water into the three-necked flask, stir, add 20g of rearrangement erythromycin A6,9-imine ether, slowly add 5% sulfuric acid to adjust the pH to 5-6, stir to dissolve, and cool down to -5°C At ~5°C, add potassium borohydride (2.50g) alkaline aqueous solution (pH=10) dropwise, keep the reaction for 3 hours after dropping, add 100ml of chloroform, adjust the pH to 10.5 with alkali, stir at 15°C to 20°C for half an hour, Stand to separate the layers, separate the chloroform, extract the water layer once with 30ml chloroform, combine the chloroform, add 200g water, cool to below 5°C, add 1g gluconic acid, wash with 20% H 2 SO 4 Adjust pH=2.8, stir and keep warm for 30min. Use 20% sodium hydroxide solution to adjust the pH to 10-11, stir for 30 minutes, let stand to separate the layers, separate the chloroform, extract the water layer with 30ml of chloroform once, combine the chloroform, add 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com