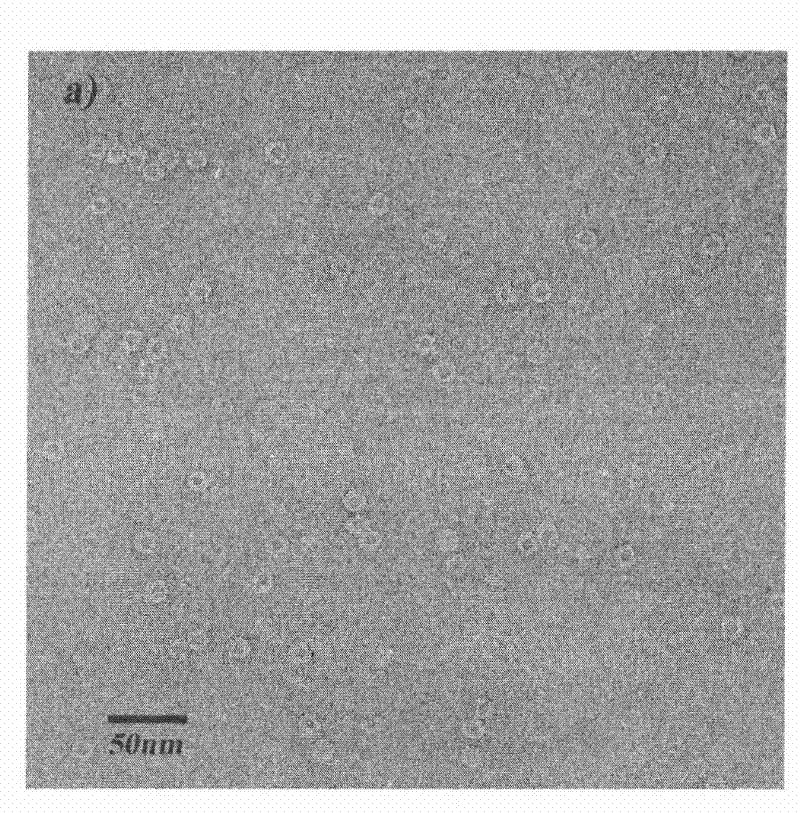
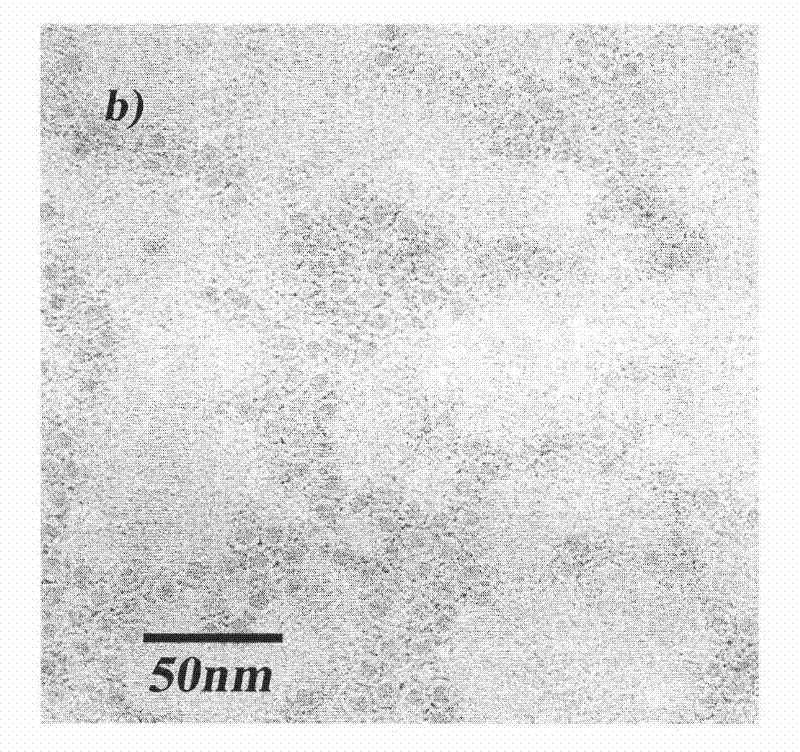
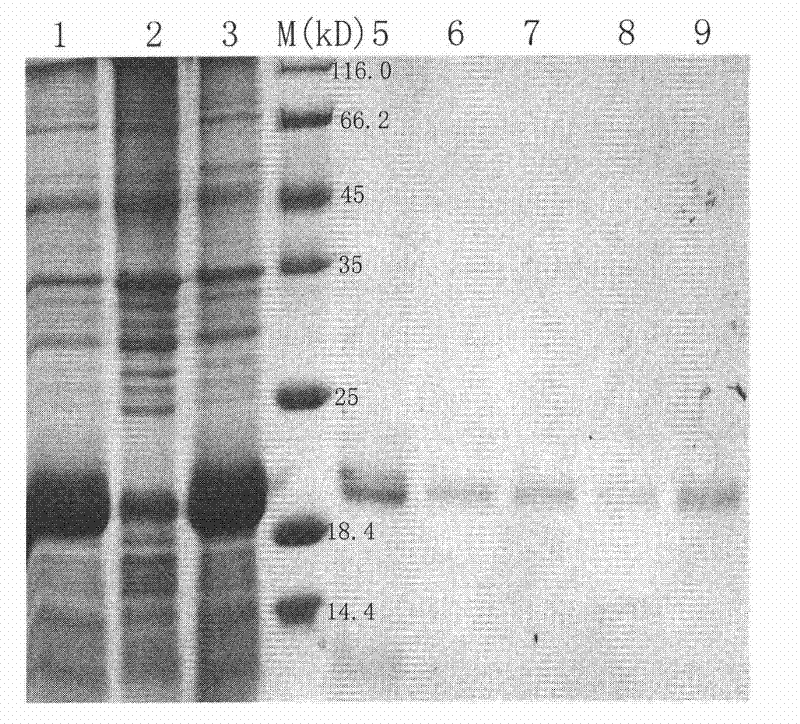
Method for separating purified ferritins from biological tissues or engineering bacteria
A biological tissue, separation and purification technology, applied in the field of protein purification, can solve the problems of low yield, high instrument requirements, cumbersome steps, etc., and achieve the effects of low raw material cost, high loading, and fast and simple purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1) Raw materials: buy 1-2 fresh pig spleens in the market, dark red in color, rich in ferritin.
[0056] 2) Pretreatment: Use clean surgical scissors to remove connective tissue such as the white fascia on the ventral surface of the spleen, and then chop it into small pieces. Add deionized water in the ratio of mass 1:1.5-2. Under the protection of low-temperature ice water, use a tissue grinder to grind the spleen tissue into a homogenate at high speed for about 20-30 minutes. Use 10 layers of gauze to filter out unhomogenized tissue pieces, fascia and other connective tissue and adipose tissue to obtain a homogenate.
[0057] 3) Extraction of crude protein: put the homogenate in a constant temperature water bath at 70-75°C, stir and heat-denature it for 20 minutes, and most of the non-heat-resistant protein denatured precipitates can be seen. Quickly place the homogenate in an ice bath at 4°C to room temperature, centrifuge at 10,000g at 4°C for 20-30min, and collec...
Embodiment 2
[0061]The Escherichia coli BL21 strain containing the recombinant human ferritin H submatrix plasmid was fermented and cultured in LB medium, and the cells were collected by centrifugation. The cells were mixed with deionized water, ultrasonically disrupted, the cell lysate was centrifuged at 10,000 g for 30 min at 4°C, and the supernatant was collected. The supernatant was heated and denatured in a constant temperature water bath at 70-75°C for 20 minutes, and most of the non-heat-resistant proteins were denatured and precipitated. The homogenate was quickly cooled to room temperature at 4°C, centrifuged at 10,000g for 20-30min at 4°C, and the supernatant was collected for later use. Then ammonium sulfate was added to the supernatant to precipitate protein at a ratio of 52g / 100ml, and placed in a refrigerator at 4°C for 6-12 hours. After the precipitation is complete, centrifuge at 10,000 g for 30 min at 4°C to collect the precipitate. The precipitate was dissolved with 50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com