Silicon-containing benzoxazine resin as well as preparation method and application thereof
A benzoxazine and benzoxazine-based technology, which is applied in the field of benzoxazine resin-silicon-containing benzoxazine monomers and polymers, can solve the problem of polycyclic benzoxazine synthesis, which has not been reported yet, etc. problems, to achieve excellent heat resistance, low surface energy, and good hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Bis(3-triethoxysilane-n-propyl-3,4-dihydro-2H-1,3-benzoxazinyl)isopropane (BZB-550)
[0079] Add 15 mL of chloroform and 3.2 g (0.1 mol) of paraformaldehyde into a 100 mL three-necked flask in sequence, and stir evenly. Dissolve 11.0g of γ-aminopropyltriethoxysilane (KH-550) (0.05mol) in 12mL of chloroform, stir evenly and add it to the paraformaldehyde solution, heat up to 85°C for 10min, add 5.7g ( 0.025mol) bisphenol A, reflux for 3h. The generated water was removed by liquid separation, and the solvent was removed by rotary evaporation to obtain a colorless and transparent liquid with a yield of 84%.
[0080] Elemental Analysis: C 37 h 62 N 2 o 8 Si 2 , % of theory: C, 61.8%; H, 8.69%; N, 3.9%. Analytical values: C, 62.1%; H, 8.44%; N, 3.84%.
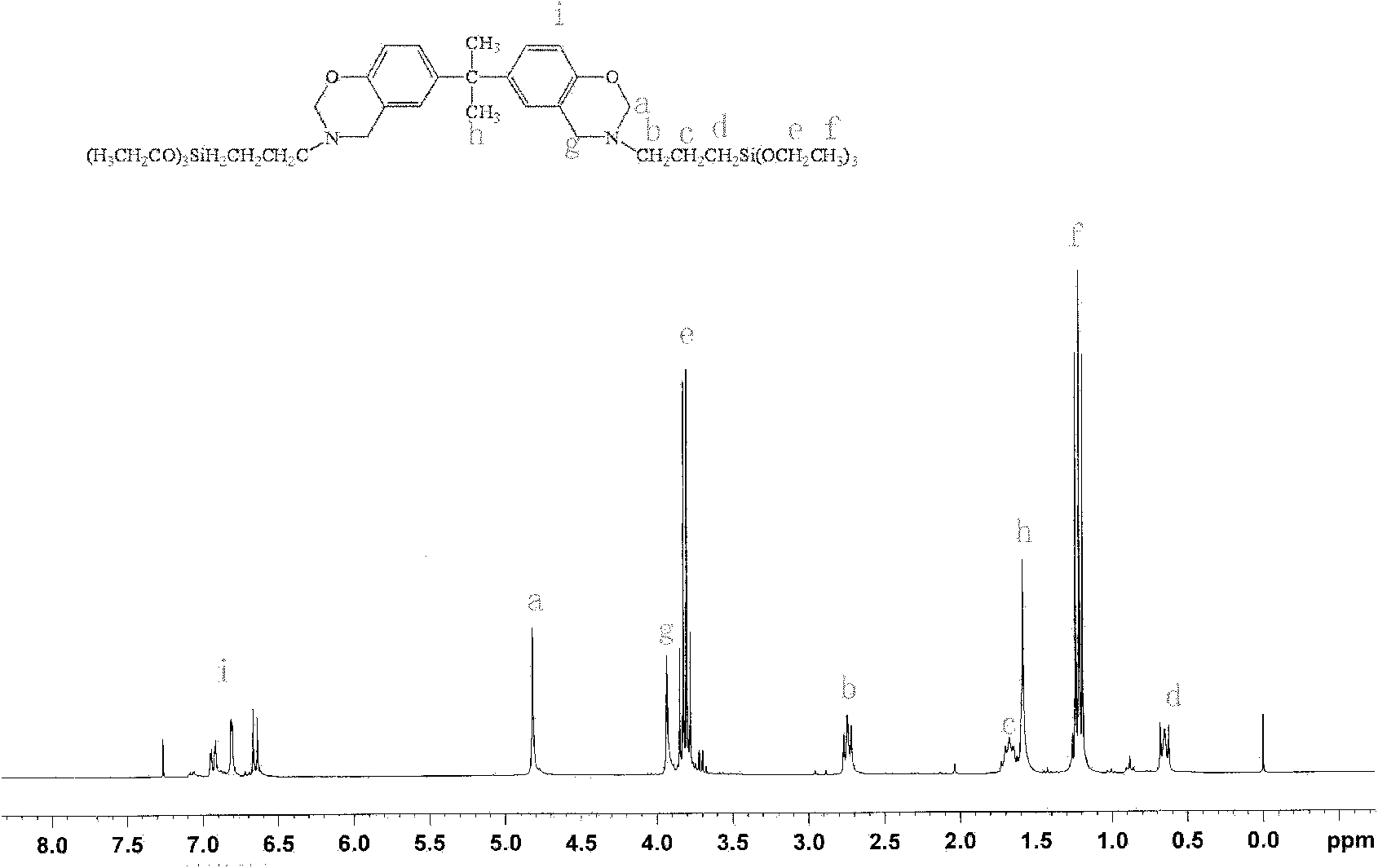
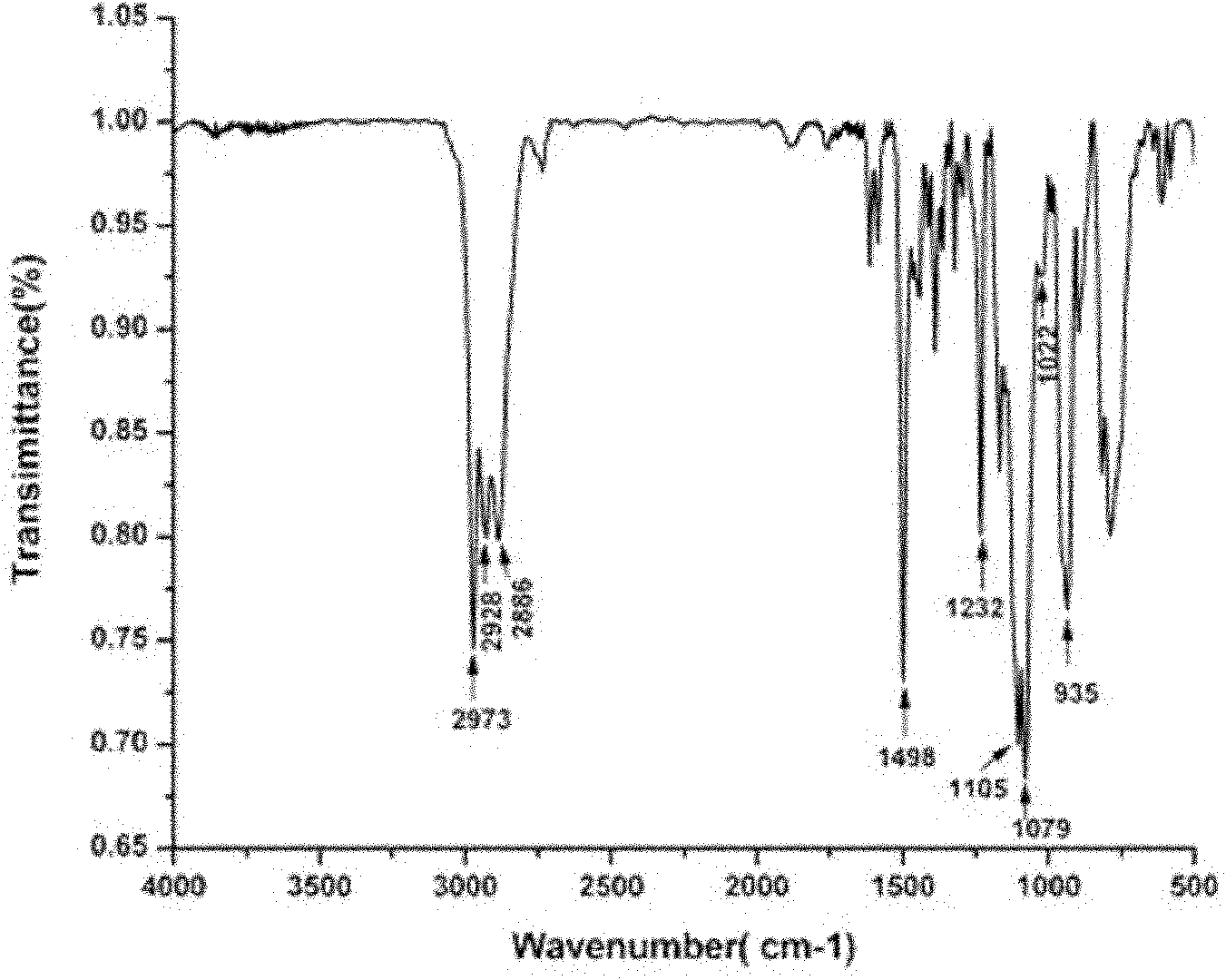
[0081] That 1 H NMR spectrum as figure 1 As shown, the FTIR spectrum is as figure 2 shown.
[0082] 1 H NMR (CDCl 3 , 300MHz, δ): 3.93ppm (s, Ar-CH 2 -N), 4.82ppm (s, O-CH 2 -N), 1.59ppm(-C(CH 3 )...
Embodiment 23
[0083] Example 23-(Methyl-dimethoxysilane)-n-propyl-3,4-dihydro-2H-1,3-benzoxazine (BZS-660)
[0084] 15 mL of dioxane and 3.2 g (0.1 mol) of paraformaldehyde were successively added into a 100 mL three-necked flask, and stirred evenly. Dissolve 8.16g of γ-aminopropylmethyldimethoxysilane (KH-660) (0.05mol) in 12mL of dioxane, stir evenly and add it to the paraformaldehyde solution, raise the temperature to 90°C for 10min, add 4.7g (0.05mol) of phenol was refluxed for 3h. The generated water was removed by liquid separation, and the solvent was removed by rotary evaporation to obtain a colorless and transparent liquid with a yield of 86%.
[0085] Elemental Analysis: C 14 h 23 NO 3 Si, theoretical %: C, 59.75%; H, 8.24%; N, 4.98%; Analytical values: C, 61.1%; H, 8.06%, N, 4.87%.
[0086] Its FTIR spectrum is as Figure 4 shown.
[0087] 1H NMR (CDCl3, 300MHz, δ): 3.95ppm (s, Ar-CH2-N), 4.86ppm (s, O-CH2-N), 2.64-2.769ppm (t, N-CH2-C), 1.60- 1.62(m, C-CH2-C), 0.68-0.74(...
Embodiment 3
[0088] Example 3 Bis(3-methyl-dimethoxysilane-n-propyl-3,4-dihydro-2H-1,3-benzoxazinyl)isopropane (BZB-660)
[0089] Add 15 mL of chloroform and 7.8 mL (0.1 mol) of formaldehyde solution to a 100 mL three-neck flask in sequence, and stir evenly. Dissolve 8.16g of KH-660 (0.05mol) in 12mL of chloroform, stir evenly and add it to the paraformaldehyde solution, heat up to 85°C for 10min, add 5.7g (0.025mol) of bisphenol A, and reflux for 3h. The generated water was removed by liquid separation, and the solvent was removed by rotary evaporation to obtain a colorless and transparent liquid with a yield of 82%.
[0090] Elemental Analysis: C 31 h 50 N 2 o 6 Si 2 , Theoretical %: C, 61.76%; H, 8.36%; N, 4.65%; Analytical value: C, 61.62%; H, 8.246%; N, 4.78%.
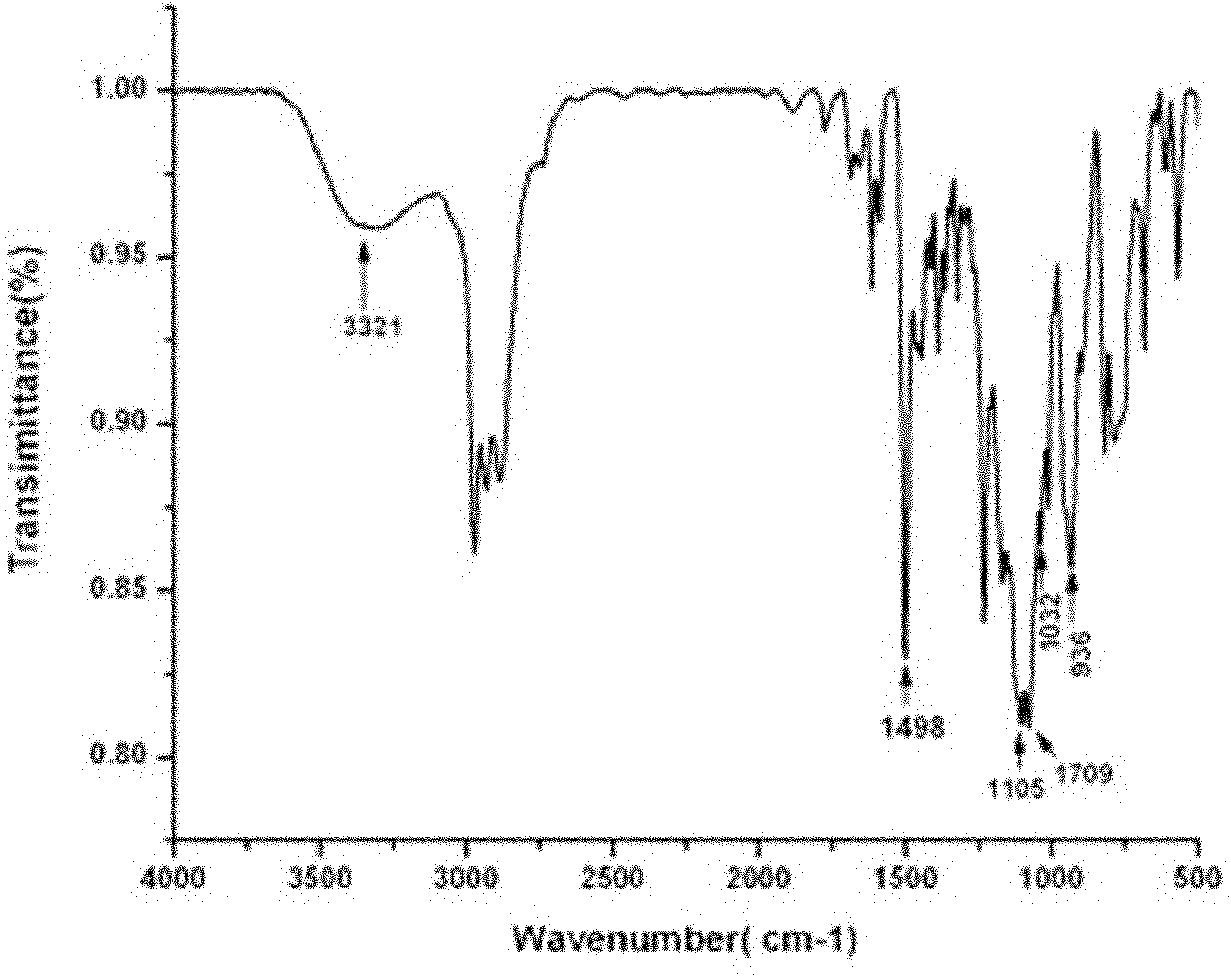
[0091] Its FTIR spectrum is as Figure 5 shown.
[0092] 1 H NMR (CDCl 3 , 300MHz, δ): 3.95ppm (s, Ar-CH 2 -N), 4.86ppm (s, O-CH 2 -N), 1.59ppm(-C(CH 3 ) 2 -), 2.64-2.769ppm (t, N-CH 2 -C), 1.60-1.62(m, C-CH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com