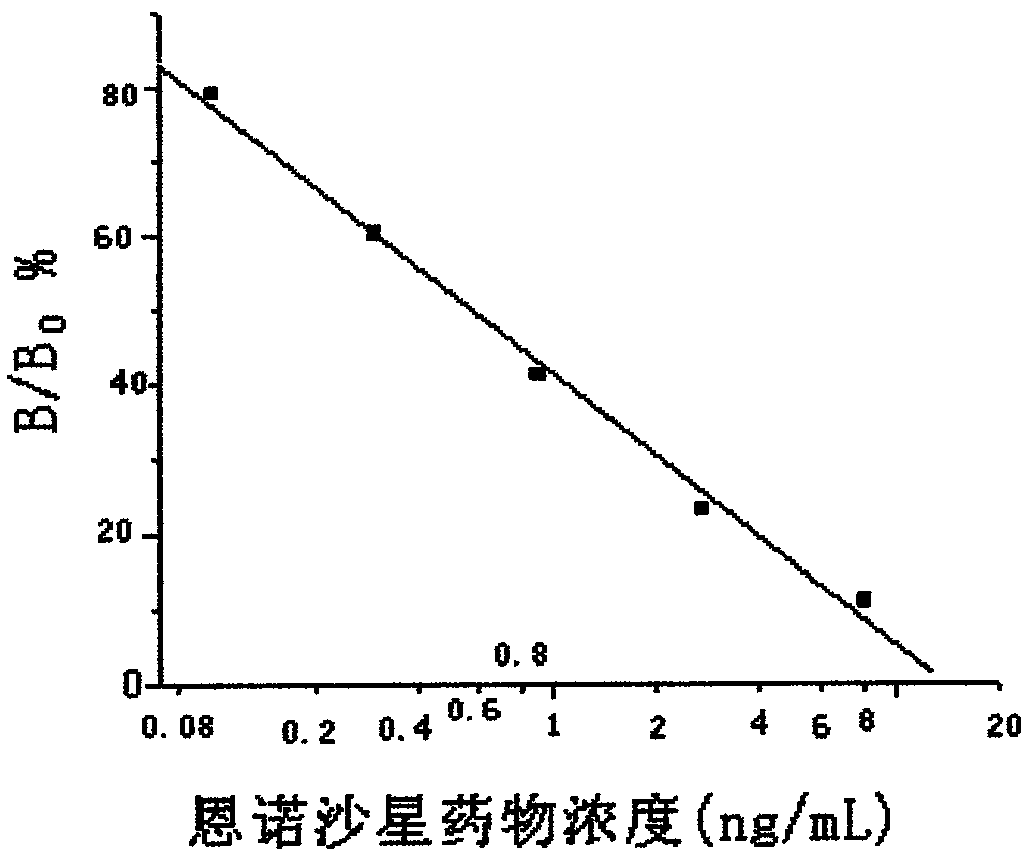
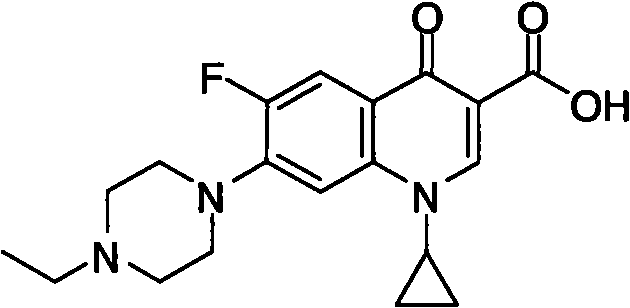
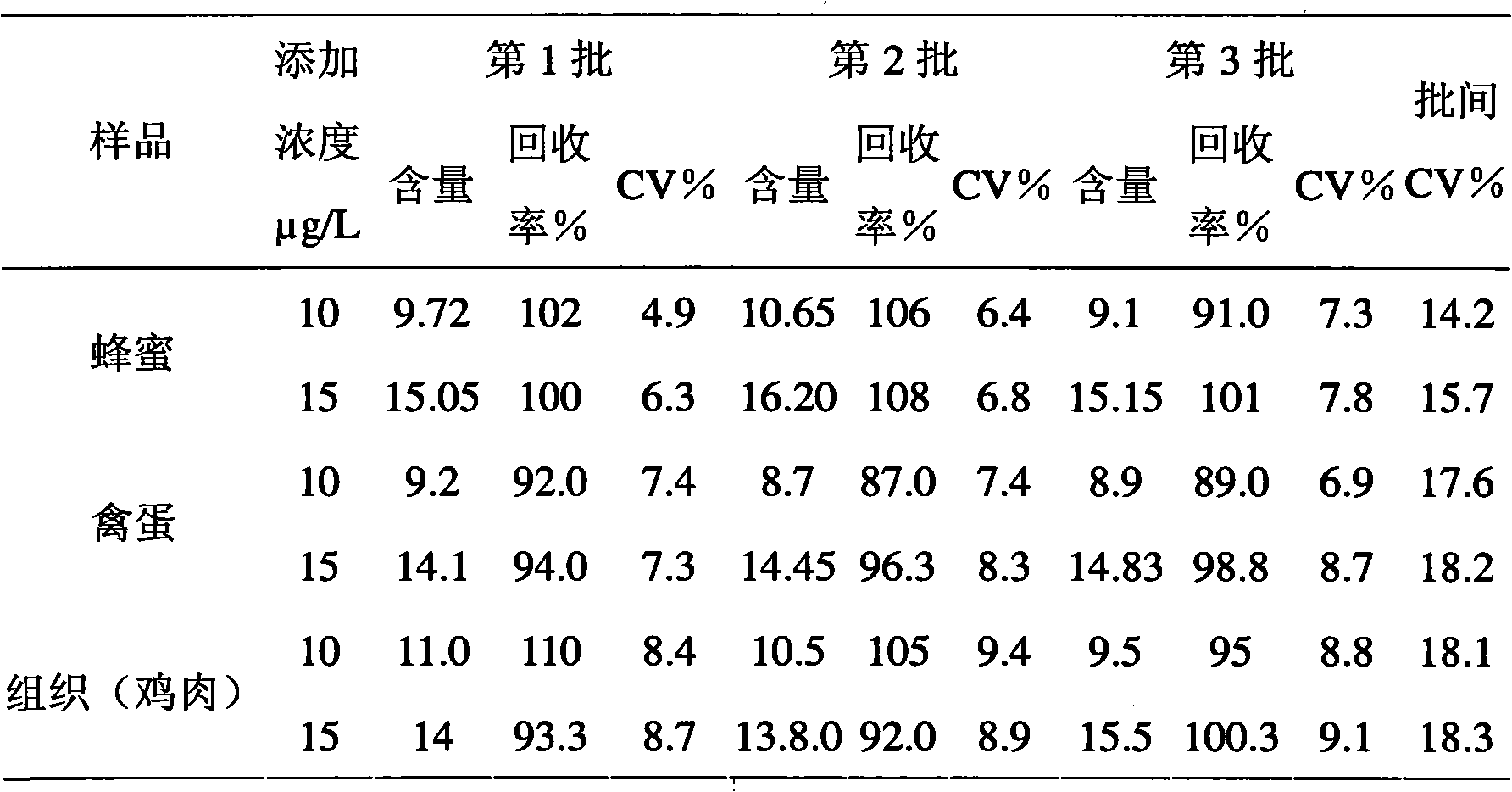
Time-resolved immunofluorometric assay detection kit for enrofloxacin residue and detection method thereof
A detection kit, the technology of enrofloxacin, applied in the field of immunodetection, can solve the problem of no kit, etc., and achieve the effects of simple sample pretreatment, good effect, and short operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 enrofloxacin antigen
[0064] a. Dissolve 50 μmol / L enrofloxacin in 1 mL of dimethylformamide (DMF), then add equimolar dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide to the solution Amine (NHS), react overnight at room temperature;
[0065] b. Centrifuge, take 800 μL of supernatant, slowly add to 4 mL of 15 mg / mL BSA or OVA carrier protein carbonate buffer solution, and then react for 4 hours under magnetic stirring;
[0066] c. After the reaction is completed, put it into a dialysis bag, first dialyze twice with distilled water, and then dialyze with 0.8% normal saline to obtain the product;
[0067] d. The binding ratio was measured by ultraviolet scanning (Chen Xinxin et al., 1998). Finally, the antigen was concentrated or lyophilized to obtain the enrofloxacin immunogen and the coating agent, which were stored in a refrigerator at -20°C.
Embodiment 2
[0068] The preparation of embodiment 2 antibody
[0069] (1) Enrofloxacin rabbit polyclonal antibody preparation
[0070] Animal immunization procedure: New Zealand white rabbits were used as immunized animals. Enrofloxacin and bovine serum albumin conjugates were used as the immunogen, and the immunization dose was 100 μg / monkey. At the first immunization, the immunogen was mixed with the same amount of Freund's complete adjuvant to make an emulsifier, and the intraperitoneal injection was performed at intervals of 3 Take the same dose of immunogen plus the same amount of Freund's incomplete adjuvant mixed and emulsified every week, and boost the immunization once, take rabbit blood, centrifuge, take the supernatant, use caprylic acid-ammonium sulfate method to purify the antibody, and freeze it in 100 μL / tube.
[0071] (2) Enrofloxacin rabbit monoclonal antibody preparation
[0072] Animal immunization: Balb / c mice were immunized at intervals with the conjugate of enroflox...
Embodiment 3
[0125] Example 3Eu 3+ Preparation and purification of labeled goat anti-rabbit
[0126] Take 1ml of 5mg / mL goat anti-rabbit IgG dissolved in 0.05mol / L PBS pH7.4, and switch the buffer salt condition through a PD-10 column, and the eluent is 50mmol / L pH 9.6NaCO 3 -NaHCO 3 buffer. The protein peaks were collected and quantified by UV absorption analysis (1.46A 280 -0.74A 260 ), dilute goat anti-rabbit to 2mg / mL with the eluent above. Take 500 μL of diluted goat anti-rabbit IgG and add 0.2 mg of Eu 3+ -N 2 -[p-isocyanic acid-benzyl]-diacetyltriaminetetraacetic acid (Eu 3+ -DTTA) in brown vials, placed in a constant temperature oven at 28°C to react for 48h, the reaction solution was chromatographed on Sepharose CL-6B (1×30cm) equilibrated with 50mmol / L Tris-HCl pH 7.8 buffer solution, and the fluorescence count of each tube was measured value, detect the first counting peak after dilution, and prepare for use after dilution.
[0127] Labeling rate = number of markers / num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com