Alprostadil lipid nanosphere injection and preparation method thereof
A technology of dil lipid and alprostadil, which is applied in blood diseases, pharmaceutical formulations, emulsion delivery, etc., can solve problems affecting the clinical application of products, increase blood circulation time, and shorten pharmacological action time, so as to reduce pulmonary circulation. Inactivation and blood clearance, reduced blood clearance, reduced effect of pulmonary circulation inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
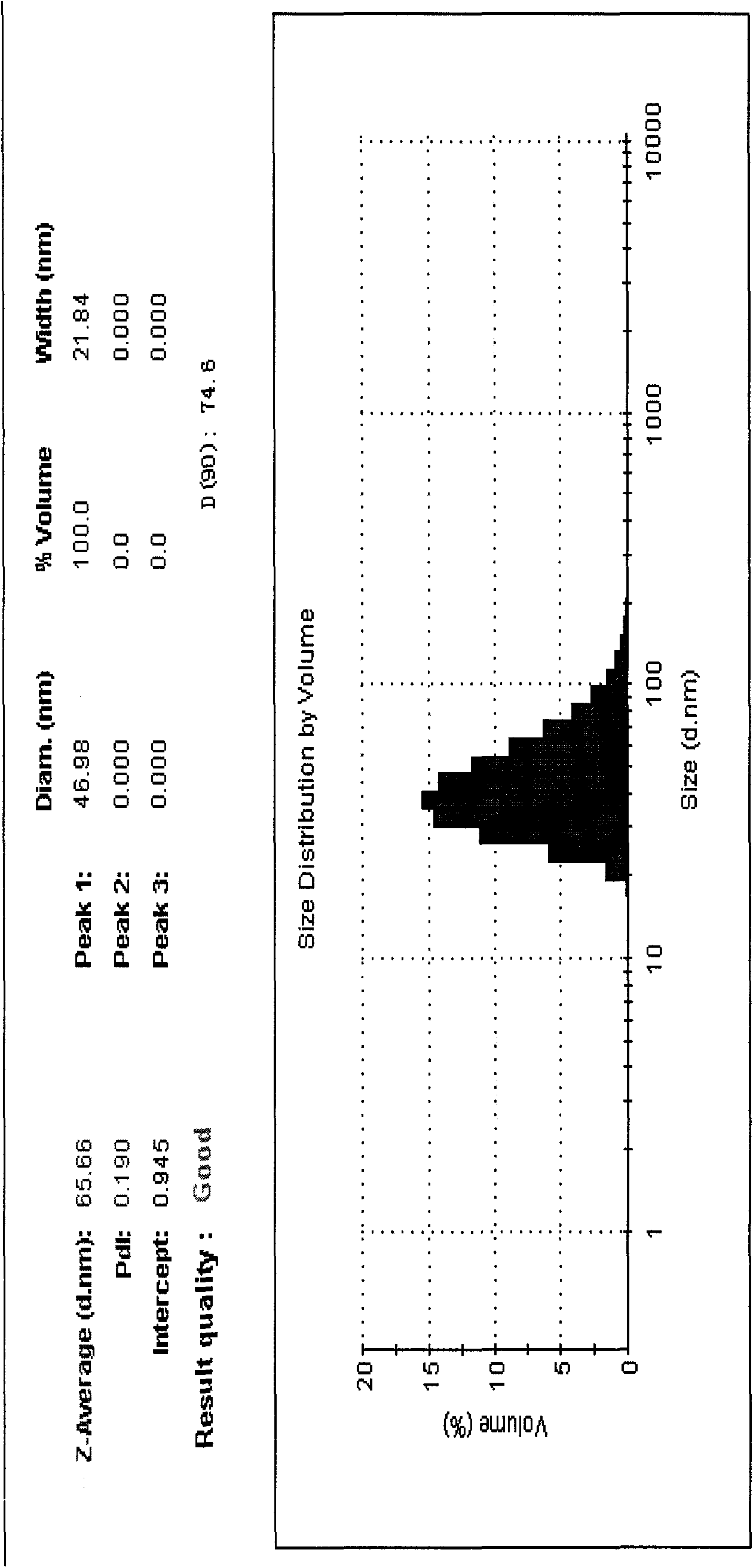
[0034] Alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials in parts by weight: 0.03 g of alprostadil, 90 g of medium-chain oil, 40 g of soybean lecithin, polyethylene glycol dodecahydroxy hard Prepared from 50g of fatty acid ester, 20g of glycerin and 800g of water.
[0035] In a sterile workshop or a 100-class purification workshop, weigh 90g of medium-chain oil for injection, add 40g of sterile soybean lecithin and 50g of sterile polyethylene glycol lauryl hydroxystearate into it, and heat it to 60 Mix well at -70°C, then add 30mg of the main drug alprostadil to dissolve it; add 800g of water for injection at 60-70°C and 20g of injection-grade glycerin, keep warm at 60-70°C, and shear and stir to form The colostrum is then placed in a high-pressure homogenizer, circulated 3-4 times under a pressure of 20-100 MPa, cooled to room temperature, filtered through a 0.45 μm filter membrane, and then filtered through a 0.22 μm...
Embodiment 2
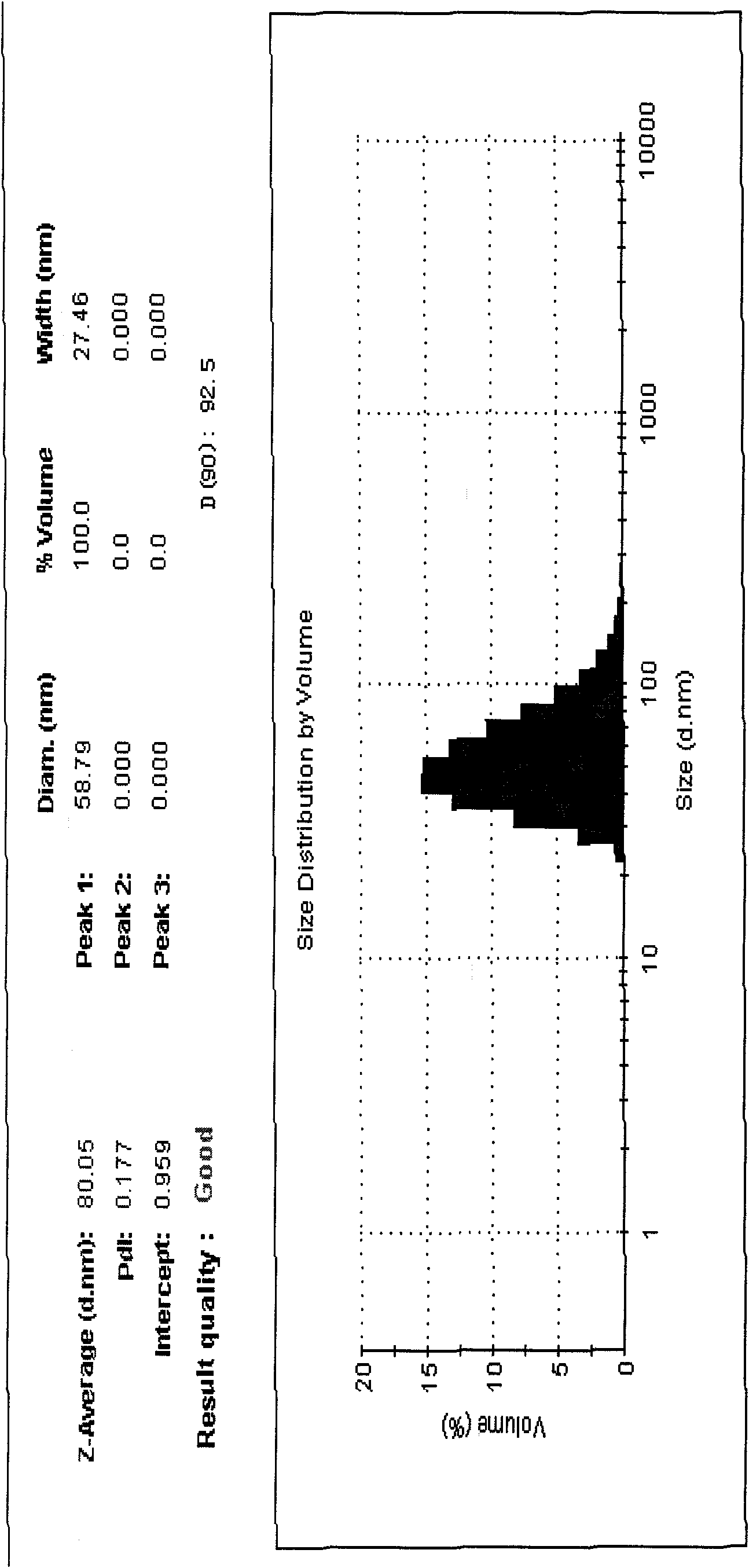
[0037] Alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials according to the ratio of parts by weight: 0.002g of alprostadil, 40g of medium chain oil, 26g of egg yolk lecithin, polyethylene glycol dodecahydroxy hard Fatty acid ester 25g, glycerin 22g, water 890g.
[0038] In a sterile workshop or a 100-grade purification workshop, weigh 40g of medium-chain oil for injection, add 26g of sterile egg yolk lecithin and 25g of sterile polyethylene glycol lauryl hydroxystearate into it, and heat it to 60 -70°C, mix well, then add 2mg of the main drug alprostadil to it, make it dissolve, keep warm at 60-70°C; add 890g of water for injection at 60-70°C and 22g of injection-grade glycerin, keep warm to 60-70°C ℃, shear and stir to form colostrum, then put it into a high-pressure homogenizer, cycle 8 times under a pressure of 20-100MPa, cool to room temperature, filter with a 0.45μm filter membrane, and then filter with a 0.22μm fi...
Embodiment 3
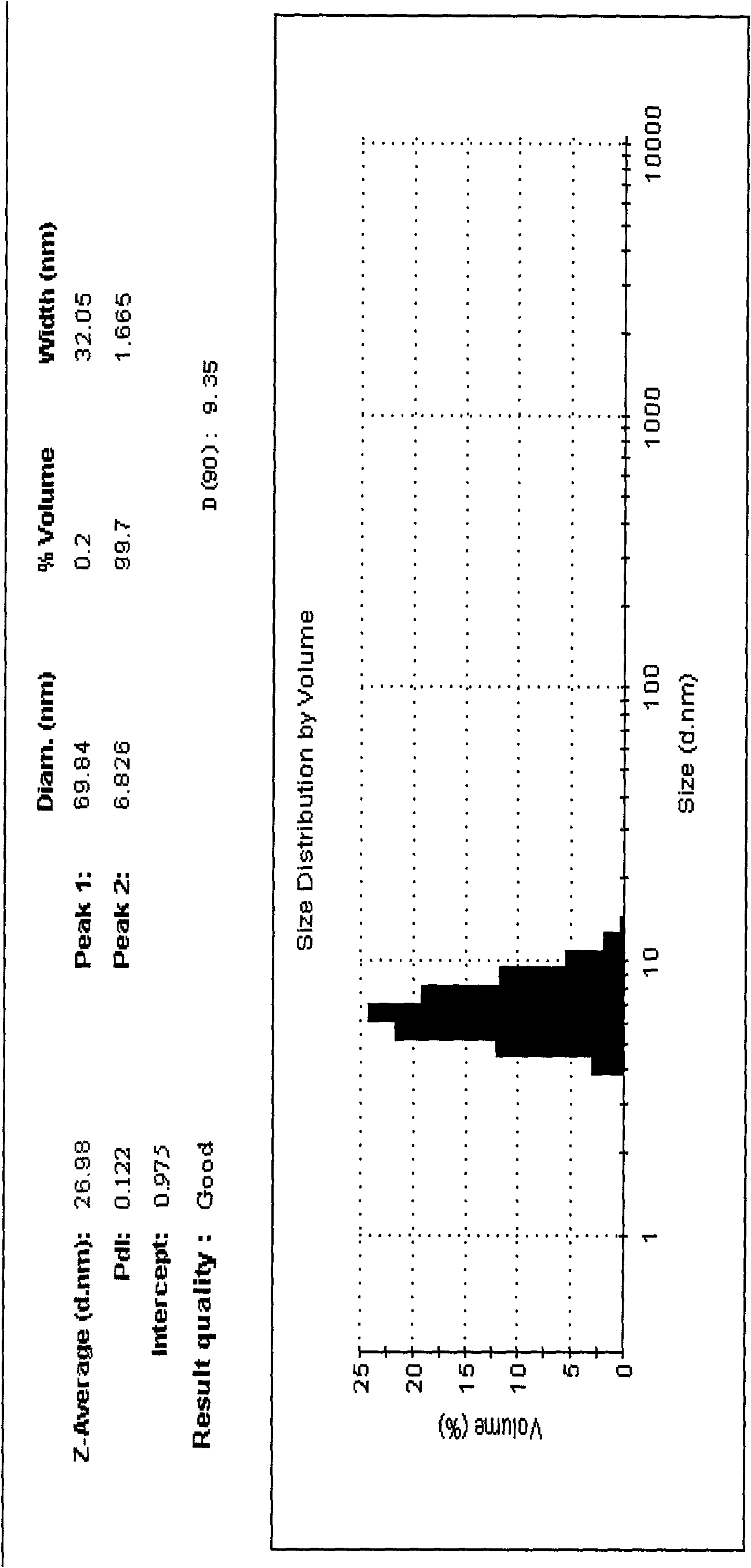
[0040] Alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials in parts by weight: 0.06 g of alprostadil, 42 g of medium chain oil, 28 g of soybean oil, 30 g of soybean lecithin, polyethylene glycol Lauryl hydroxystearate 40g, glycerin 22g, water 838g.
[0041] In a sterile workshop or a 100-grade purification workshop, weigh 42g of medium-chain oil for injection and 28g of soybean oil for injection according to the weight ratio, and add 30g of sterile soybean lecithin and sterile polyethylene glycol dodecahydroxyl to it. Stearate 40g, heat it to 60-70°C, mix well, then add 60mg of the main drug alprostadil to it, dissolve it, keep warm at 60-70°C; add 60-70°C water for injection 838g and injection grade The mixture of 22g glycerin is kept at 60-70°C, sheared and stirred to form colostrum, then placed in a high-pressure homogenizer, circulated 10 times under a pressure of 20-100MPa, cooled to room temperature, and filtered t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com