Controlled-release implanting preparation used for injecting LHRH (luteinizing hormone releasing hormone) antagonist
An antagonist and injection technology, applied in the field of sustained-release implant preparations, can solve the problems of increasing the dosage, affecting the penetration and diffusion of chemotherapy drugs, and difficult to form effective drug concentrations locally in tumors, so as to reduce systemic toxic reactions and prolong Effect of Local Drug Concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 10% Cetrorelix Acetate, 90% PLGA. Its preparation process is as follows:
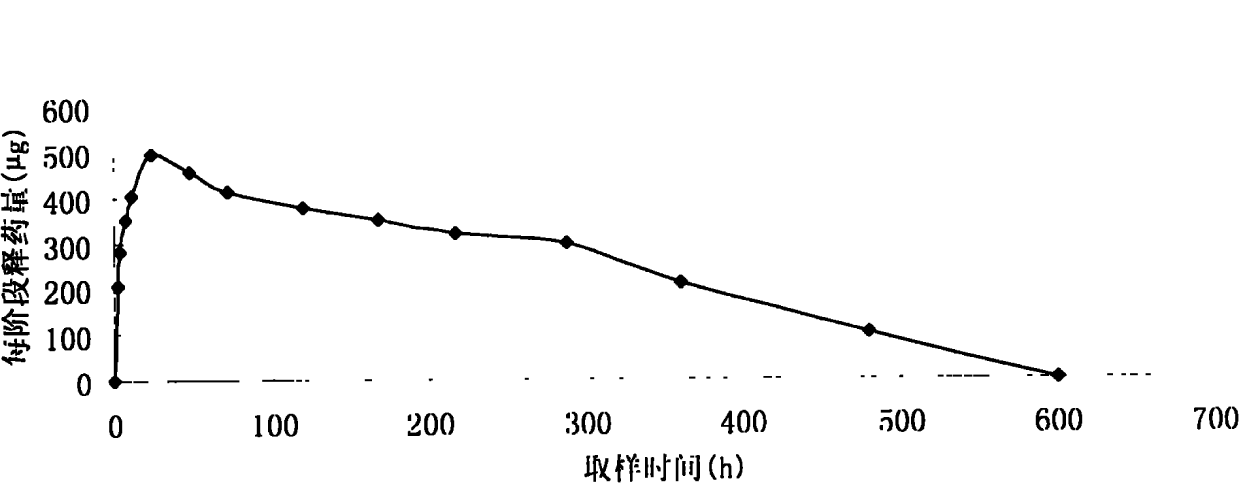
[0019] Accurately weigh 600 mg of cetrorelix acetate, add 150 ml of acetone to a stoppered Erlenmeyer flask to dissolve it; precisely weigh 5400 mg of PLGA, add 150 ml of acetone to dissolve, mix the above two solutions, place them overnight at room temperature, and vortex to make It is fully mixed evenly, and the solution is a viscous transparent liquid. Pour it several times onto a clean horizontal glass template, heat in water at 40°C for 24 hours to fully volatilize the acetone, and form a white drug-containing PLGA film with a thickness of about 1 mm and a certain degree of elasticity. The film was completely removed, put into a mold, pressed into a cylindrical shape on a water bath at 50°C, left at room temperature for 2 days, and freeze-dried for more than 24 hours. After aliquoting, use cobalt 60 Sterilize. The resulting implant was cylindrical, with a diameter of 0.2 cm and a length ...
Embodiment 2
[0036] 15% Cetrorelix acetate, 85% PLCG. Its preparation process is as follows:
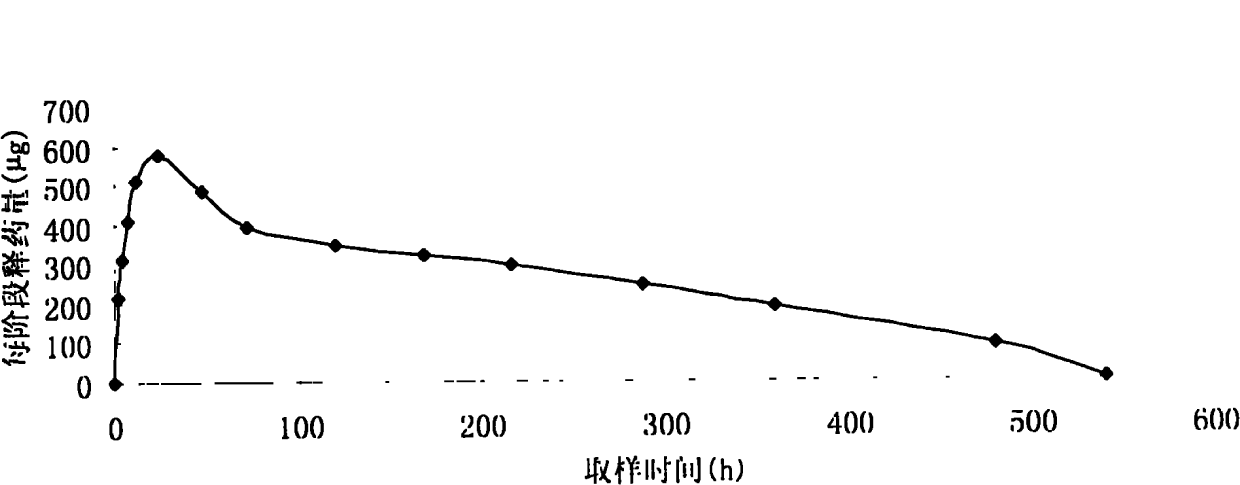
[0037] Accurately weigh 900 mg of cetrorelix acetate, add 230 ml of acetone to a stoppered Erlenmeyer flask to dissolve it; accurately weigh 5100 mg of PLCG, add 140 ml of acetone to dissolve, mix the above two solutions, and place at room temperature overnight. Vortex to make it fully mixed, the solution is a viscous transparent liquid. Pour it several times onto a clean horizontal glass template, and heat it in a water bath at 40°C for 24 hours to fully volatilize the acetone to form a white drug-containing PLCG film with a thickness of about 1 mm and a certain degree of elasticity. The film was completely removed, put into a mold, pressed into a cylindrical shape on a water bath at 50°C, left at room temperature for 2 days, and freeze-dried for more than 24 hours. After packing, drill 60 Sterilize. The resulting implant was cylindrical, with a diameter of 0.2 cm and a length of 1.0 cm.
...
Embodiment 3
[0054] 20% Cetrorelix Acetate, 80% PLA. Its preparation process is as follows:
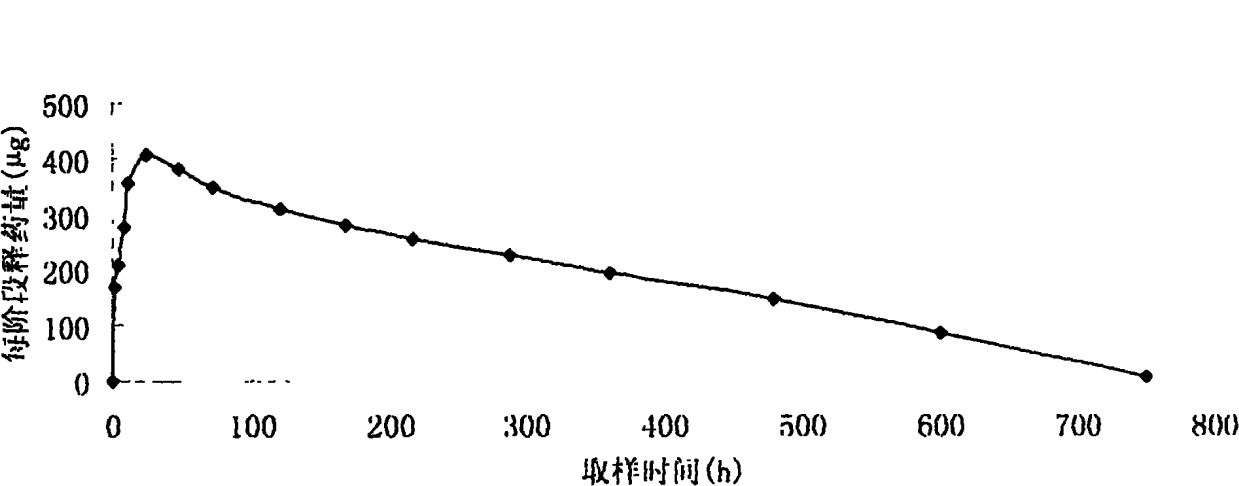
[0055] Accurately weigh 1200 mg of cetrorelix acetate, add 300 ml of acetone to a stoppered Erlenmeyer flask to dissolve it; accurately weigh 4800 mg of PLA, add 100 ml of acetone to dissolve, mix the above two solutions, and place at room temperature overnight. Vortex to make it fully mixed, the solution is a viscous transparent liquid. Pour it several times onto a clean horizontal glass template, and heat it in a water bath at 40°C for 24 hours to fully volatilize the acetone to form a white drug-containing PLA film with a thickness of about 1 mm and a certain degree of elasticity. The film was completely removed, put into a mold, pressed into a cylindrical shape on a water bath at 50°C, left at room temperature for 2 days, and freeze-dried for more than 24 hours. After packing, drill 60 Sterilize. The resulting implant was cylindrical, with a diameter of 0.2 cm and a length of 1.0 cm.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com