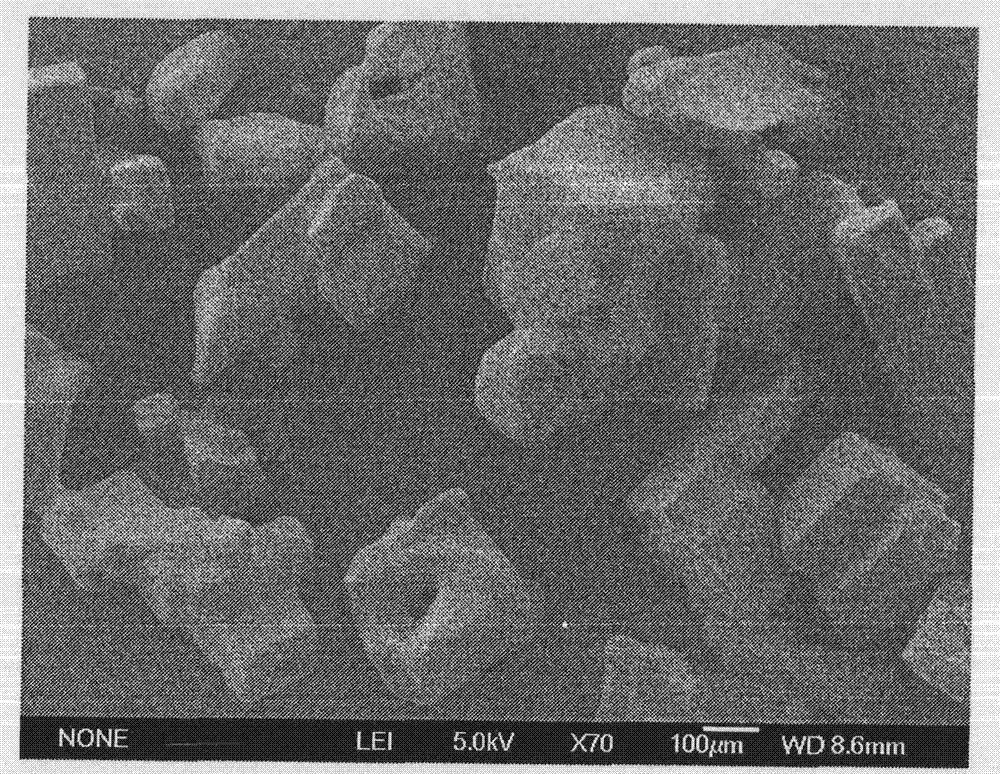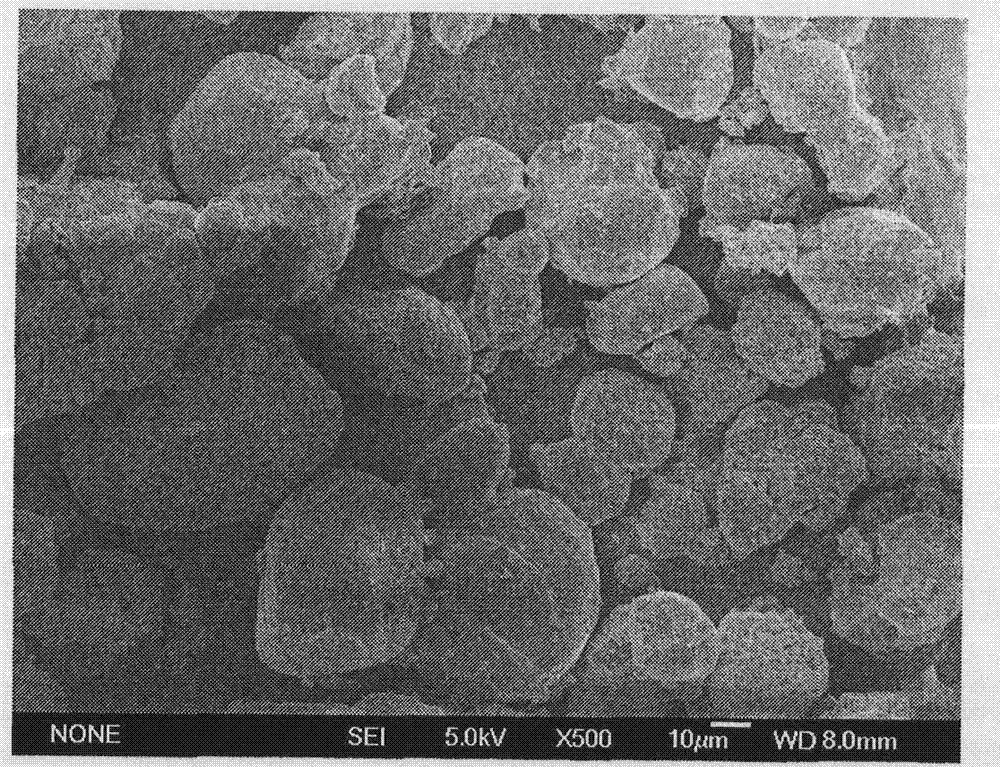Method for recovering ammonium chloride by preparing complex salt of magnesium ammonium chloride hexahydrate
A technology of ammonium chloride hexahydrate and ammonium chloride, which is applied in the direction of ammonium halide and chloride preparation, etc., to achieve the effects of low energy consumption, reduced environmental impact, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
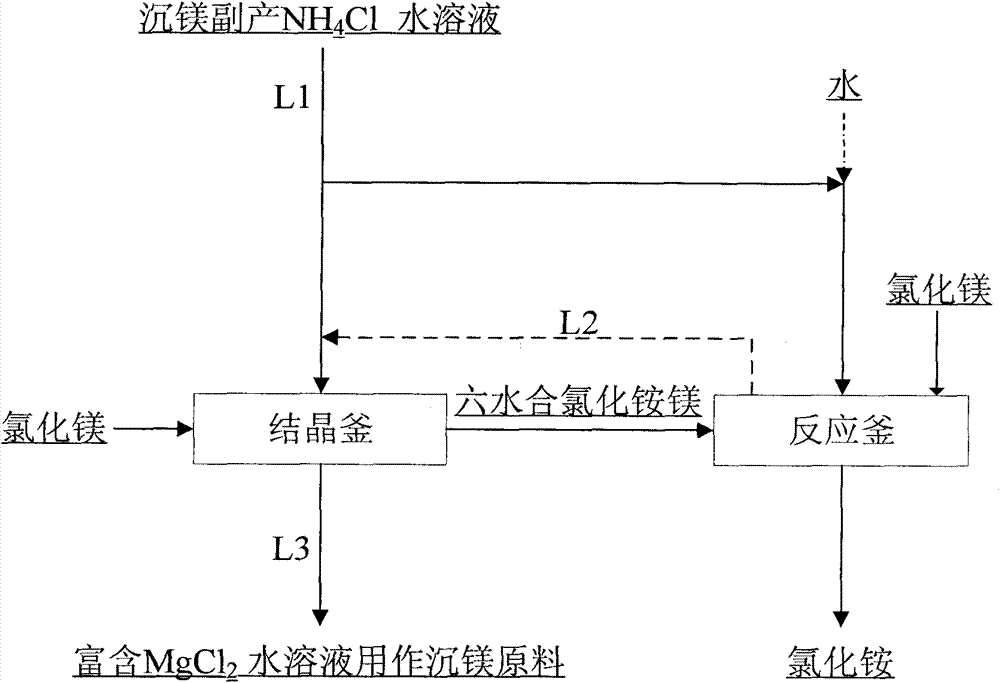
[0029] For the specific process flow of this embodiment, please refer to figure 1 .
[0030] The raw material ammonium chloride solution selected in this embodiment is a by-product in the magnesium precipitation process. Concentration is 6mol / L; Add 400ml of ammonium chloride solution into the crystallization kettle, adjust the pH=4 of ammonium chloride solution in the crystallization kettle with hydrochloric acid (15wt%), then add saturated magnesium chloride brine until the concentration of magnesium chloride in the crystallization kettle reaches 3mol / L ; Under the condition of controlling the temperature of the crystallization tank at 10° C., continue stirring for 120 minutes; after filtering, the solid obtained is dried to obtain 109 g of magnesium ammonium chloride hexahydrate. According to the ratio of 0.7:1 in the mass ratio of tap water to magnesium ammonium chloride hexahydrate, the obtained 109g of magnesium chloride ammonium chloride hexahydrate is added to a react...
Embodiment 2
[0032] For the specific process flow of this embodiment, please refer to figure 1 .
[0033] Add 800ml of magnesium chloride by-product ammonium chloride solution in the ammonium bicarbonate method or ammonia magnesium precipitation process into the crystallization tank, wherein the ammonium chloride solution concentration is 1mol / L, adjust the ammonium chloride solution with hydrochloric acid (5wt%) pH = 6, then add anhydrous magnesium chloride until the concentration of magnesium chloride in the crystallization tank reaches 5mol / L; under the condition of controlling the temperature of the crystallization tank at 80°C, continue stirring for 30 minutes; the filtered solid is dried to obtain 395g of magnesium ammonium chloride hexahydrate . According to the mass ratio of the ammonium chloride solution and magnesium ammonium chloride hexahydrate by-product of heavy magnesium chloride, the 395g magnesium chloride ammonium chloride hexahydrate obtained will be added into a contai...
Embodiment 3
[0035] For the specific process flow of this embodiment, please refer to figure 1 .
[0036]400ml of by-product ammonium chloride solution by the ammonium bicarbonate method or the ammonia method of magnesium precipitation and 200ml of the filtrate obtained after the decomposition of ammonium chloride magnesium hexahydrate and recovery of ammonium chloride in Example 2 are added to the crystallization kettle together, wherein the ammonium chloride Solution concentration is 3mol / L, then ammonium chloride solution is adjusted ammonium chloride solution pH=4.5 with hydrochloric acid (36wt%), then adds magnesium chloride hexahydrate until magnesium chloride concentration reaches 3.5mol / L in the crystallization kettle; Stirring was continued for 100 minutes under the condition of 40° C.; the solid obtained after filtration was dried to obtain 256 g of magnesium ammonium chloride hexahydrate. According to the mass ratio of ammonium chloride solution and ammonium chloride magnesium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com