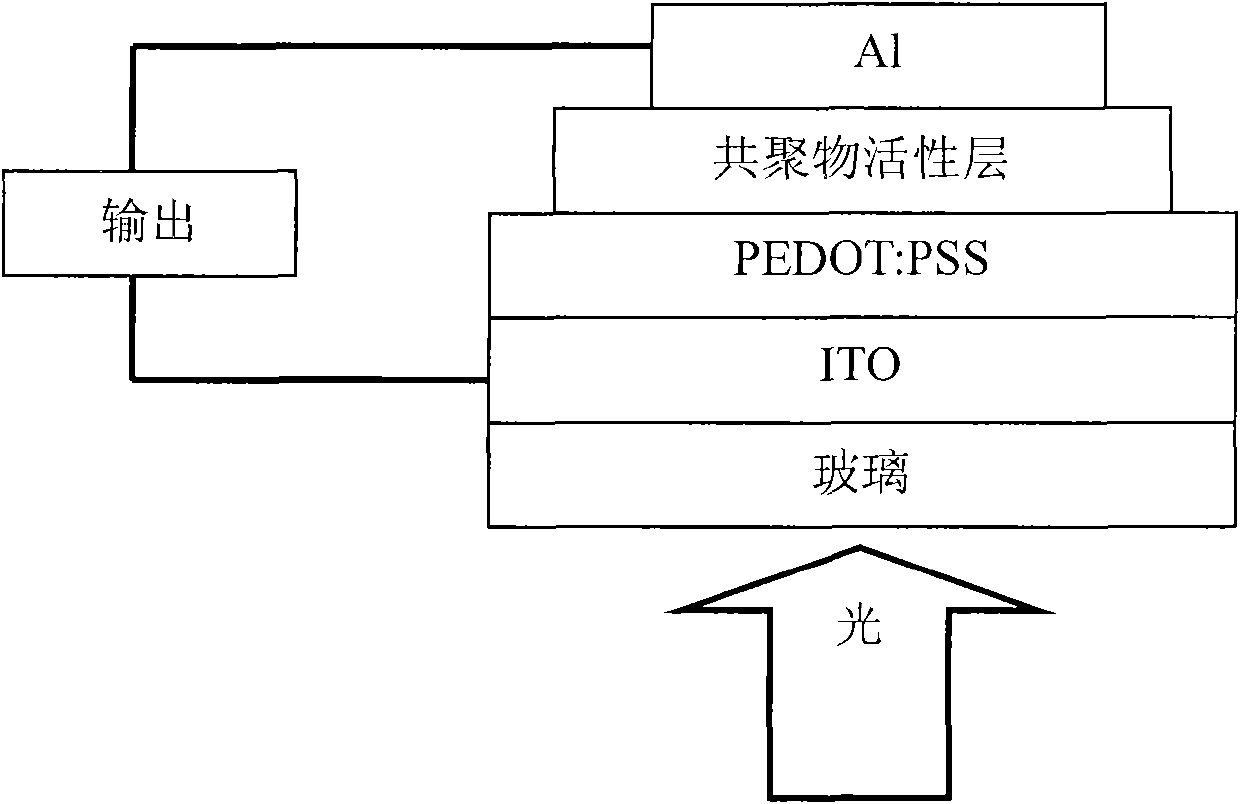
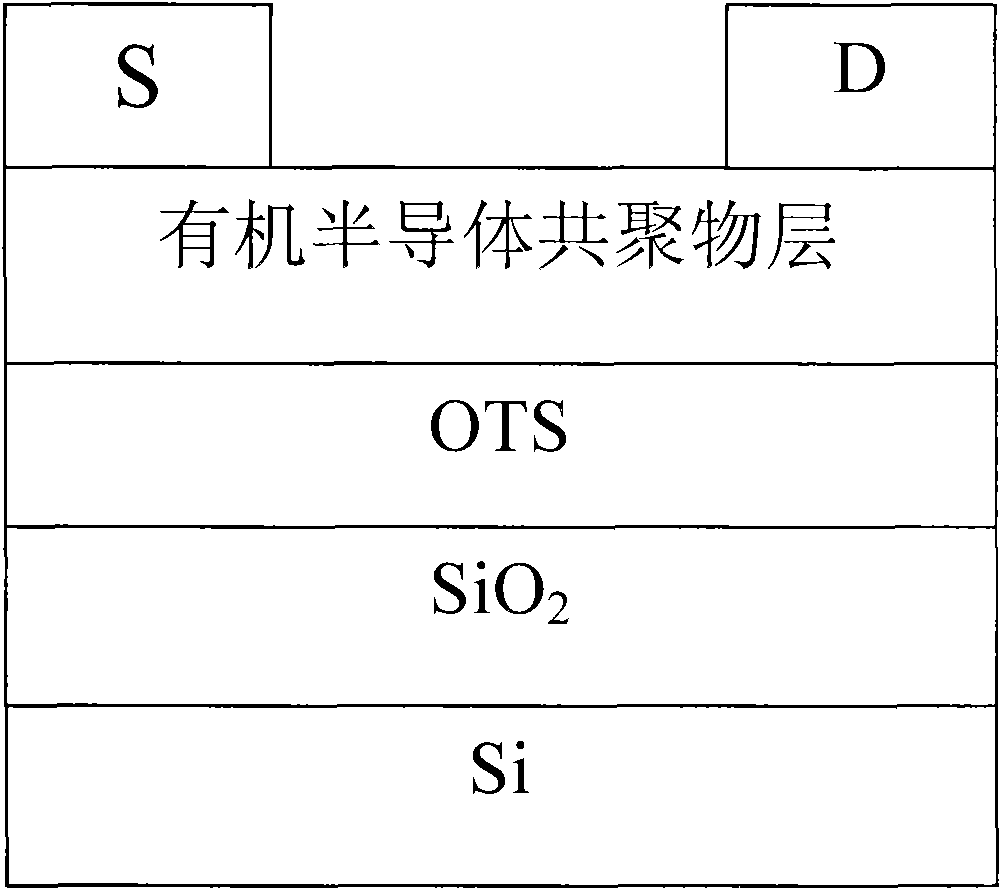
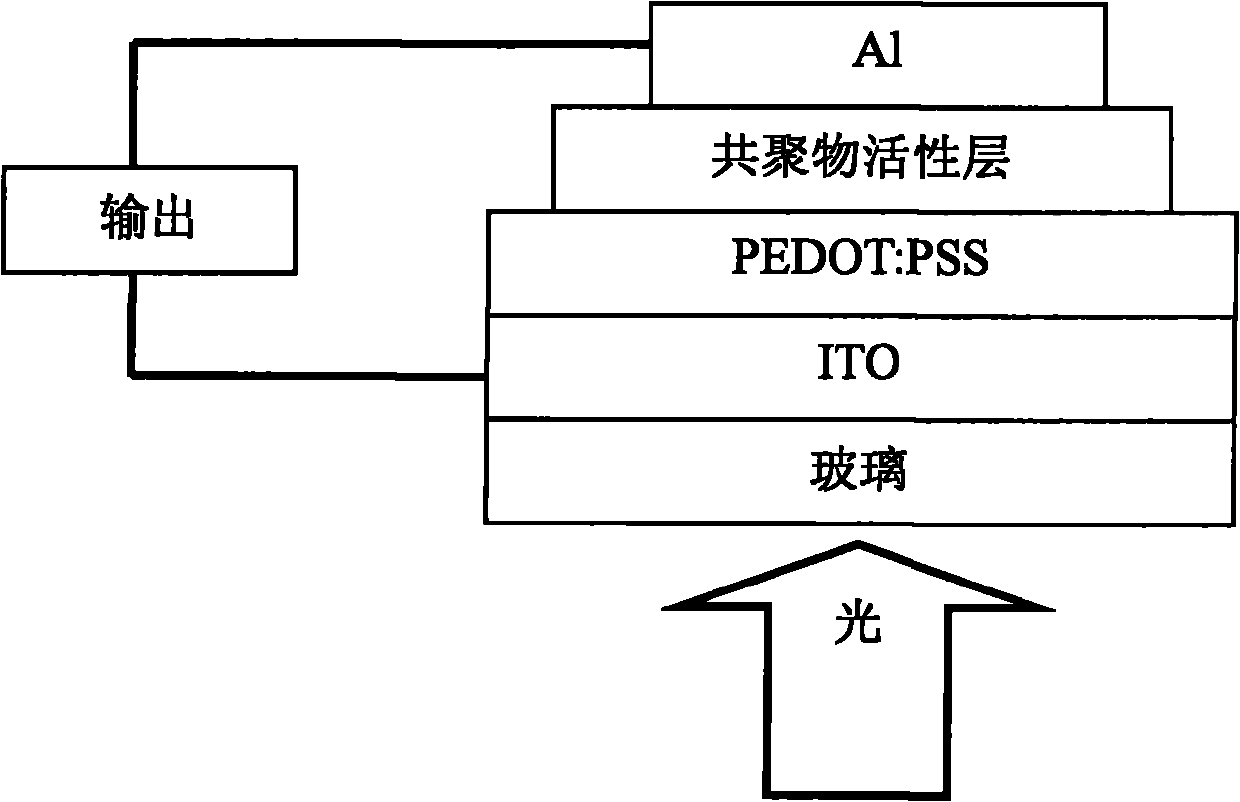
Anthracene-diazosulfide copolymer, and preparation method and application thereof
A technology of benzothiadiazoles and copolymers, which is applied in the field of organic compound synthesis, can solve problems such as limiting the scope of application, and achieve the effects of increasing the absorption range, reducing the energy gap, and being easy to operate and control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan)yl-anthracene:
[0055]
[0056] Set up an anhydrous and anaerobic reaction device, under constant stirring and N 2 Under the protection of the three-necked flask, add 7.2mmol of light yellow crystal 9,10-dibromoanthracene, and inject 120ml of tetrahydrofuran solvent, and then slowly inject 21.6mmol of n-BuLi at -78°C to react while stirring After 2 hours of reaction, the system gradually changed from light yellow to orange.
[0057] After reacting for 2 hours, inject 24.5mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane at -78°C until the system After the red color changed to pale yellow, the temperature was raised to room temperature and the reaction was continued for 12 hours.
[0058] After the reaction is over, add saturated aqueous sodium chloride solution, then extract with chloroform, then dry with anhydrous sodium sulfate, filter, then collect the filtrate and rotary evaporate the solve...
Embodiment 2
[0060] Preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan)-2,6-bis(2-octyldecyl)anthracene:
[0061]
[0062] Set up an anhydrous and anaerobic reaction device, under constant stirring and N 2 Under the protection of 9,10-dibromo-2,6-bis(2-octyldecyl)anthracene 5mmol into the three-necked flask, and inject 150ml of tetrahydrofuran solvent, then slowly inject 15mmol n -BuLi, while stirring for 2 hours, inject 15mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane at -78°C, After 52 hours of reaction, the temperature was raised to room temperature and the reaction was continued for 8 hours. After the reaction, add saturated aqueous sodium chloride, then extract with chloroform, then dry with anhydrous sodium sulfate, filter, then collect the filtrate and rotary evaporate the solvent to obtain a crude product, and finally separate the crude product by silica gel column chromatography , to obtain a powdery solid with a yield of 61%. GC-MS (EI-m / z): 959 (M+)....
Embodiment 3
[0064] Preparation of anthracene and 2,1,3-benzothiadiazole copolymer PAn-BT:
[0065]
[0066] Add 1.0 mmol of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) anthracene, 4,7-dibromo-2 to the reactor, 1,3-benzothiadiazole 1.0mmol, tetrakistriphenylphosphine palladium 0.01mmol, 2mol / L Na 2 CO 3 2.8ml of aqueous solution and 20ml of toluene solvent, through the uninterrupted flow of N 2 Keep the reaction system in an oxygen-free environment by vacuuming, and react at 94° C. for 24 hours. After the reaction is over, add deionized water and toluene to the reaction bottle for extraction, keep the organic phase, and then use the vacuum distillation method to spin-evaporate the mixed solution of the copolymer and toluene to about 5ml, and then drop it into a 300ml After being stirred continuously for about 4 hours in anhydrous methanol, flocculent solids were precipitated, and then solid powder was obtained after suction filtration and drying. Dissolve the solid powder in c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com