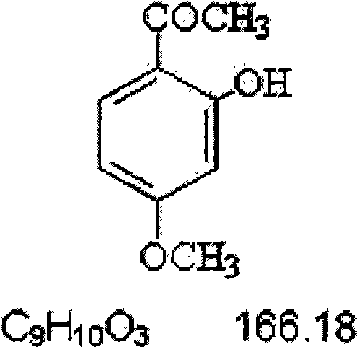New medical application of paeonol and derivatives thereof
A technology of paeonol and derivatives is applied in the field of medicine to achieve the effects of strong pharmacological action, good medicinal prospects and rich sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Paeonol Sodium Sulfonate Eye Drops: Prepare different concentrations of Paeonol Sodium Sulfonate Eye Drops according to the conventional method for preparing eye drops. The eye drops are based on Paeonol Sodium Sulfonate. active ingredient. In the preparation process, in addition to conventionally adding co-solvents, osmotic pressure regulators, antioxidants, preservatives, and pH regulators, auxiliary materials such as borneol, peppermint, and sodium hyaluronate or methylcellulose or Viscous excipients such as hydroxypropylmethylcellulose. Its purpose is to increase the concentration of the drug in the eye tissue, increase its bioavailability, enhance the curative effect and reduce adverse reactions.
[0033] The above co-solvent refers to: at least one selected from cyclodextrin, polypropylene polymer, polyvinyl alcohol, polyvinylpyrrolidone, p-aminobenzoic acid, polysorbate, or at least one of their mixtures A sort of. The osmotic pressure regulator...
Embodiment 2
[0039] Preparation of paeonol eye ointment: Paeonol eye ointment with different concentrations was prepared according to the conventional method for preparing eye ointment, and the eye ointment uses paeonol as its main active ingredient. In the preparation process, different pastes such as lanolin, yellow petrolatum, liquid paraffin, or their mixtures can be selected, and auxiliary materials such as borneol, mint, and cyclodextrin can also be optionally added. Its purpose is to increase the concentration of the drug in the eye tissue, increase its bioavailability, enhance the curative effect and reduce adverse reactions.
[0040] Concrete example: the configuration of 1% paeonol eye ointment. In 1000 grams of paeonol eye ointment, 10 grams of paeonol and 5 grams of borneol are contained, and the rest is an eye ointment base composed of yellow vaseline, lanolin and liquid paraffin.
[0041] The ratio of each component of the eye ointment base is: yellow petrolatum: lanolin: li...
Embodiment 3
[0045] Research on the therapeutic effect of the present invention on active immune conjunctivitis induced by ovalbumin.
[0046] 1. Test material
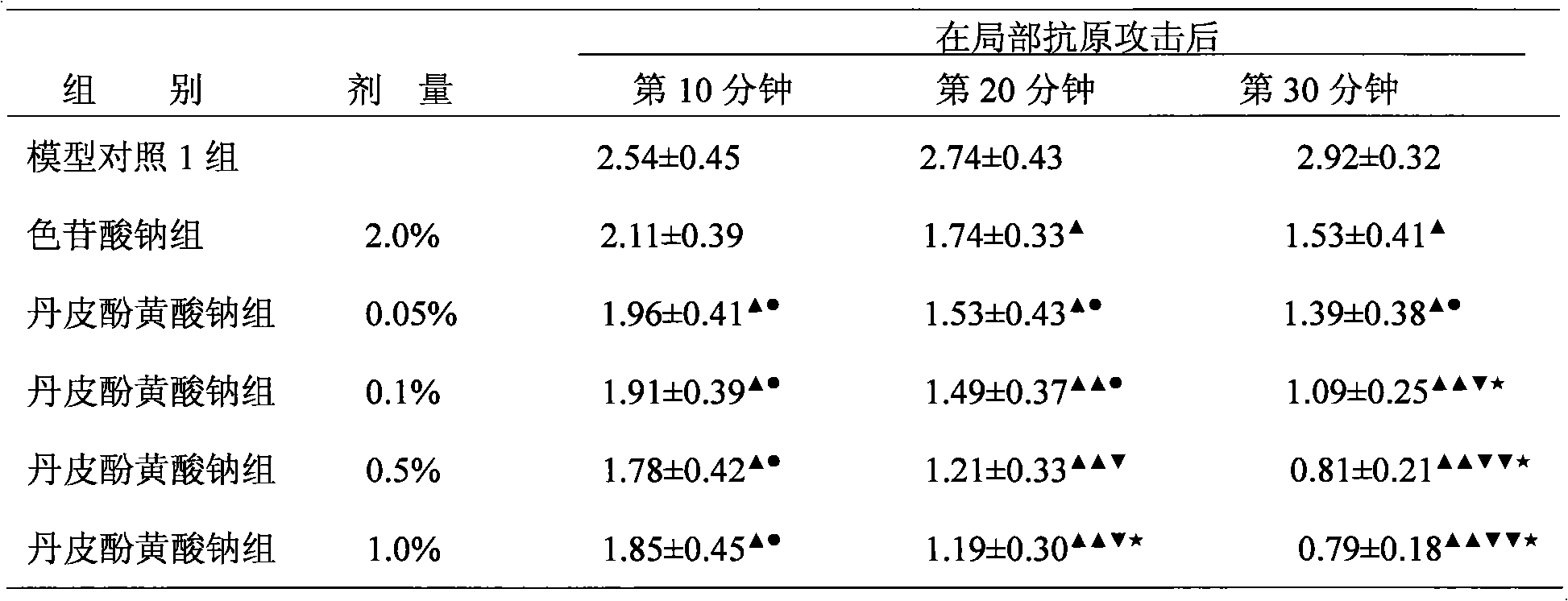
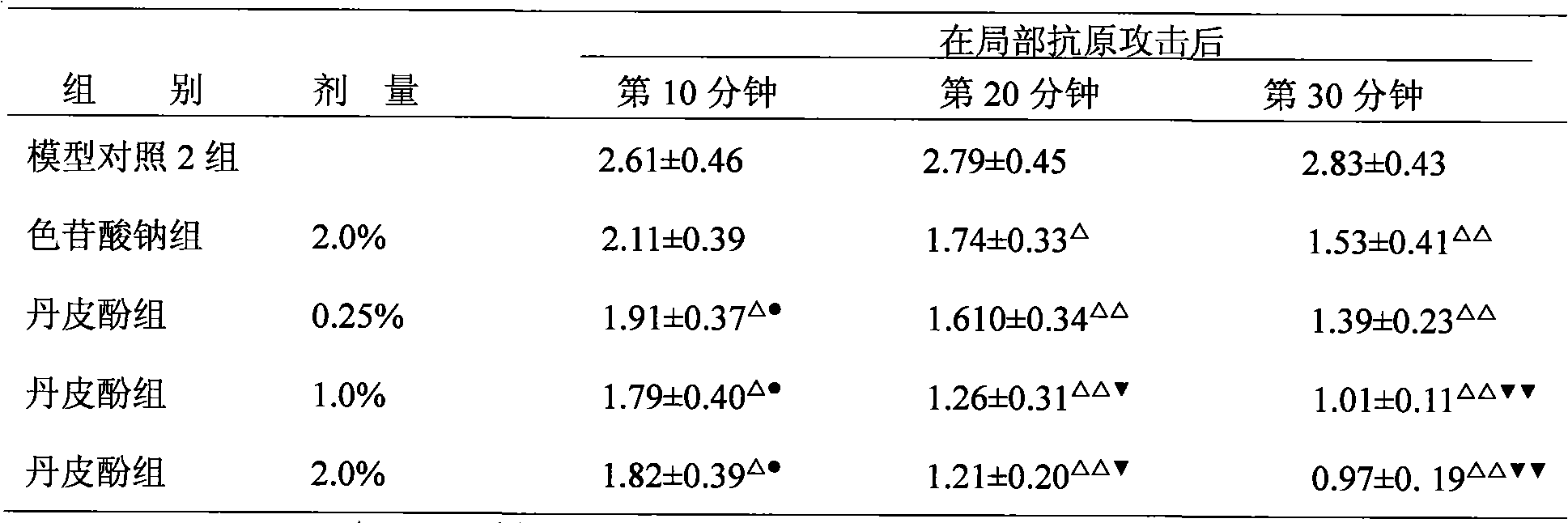
[0047] The sodium paeonol xanthate of the present invention is used to prepare 0.05%, 0.1%, 0.5%, 1% eye drops; the paeonol of the present invention is used to prepare 0.25%, 0.5%, 2.0% ophthalmic ointment. Sodium chromylate eye drops were used as the positive control group.
[0048] 2. Test method
[0049] (1) Modeling method
[0050] 50 SD rats, weighing 100g-120g, half male and half male. Using the animal model method of "active allergic conjunctivitis induced by ovalbumin" reported in open literature, rats are made as a rat model of immune (also known as allergic) conjunctivitis.
[0051] (2) Test groups
[0052] 50 rats that have been made into immune conjunctivitis animal models are randomly divided into 10 groups, with 5 animals (10 eyes) in each group: model control group 1 (vehicle group of paeonol xanthate eye drops...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com