Entamoeba histolytica galactose/acetylgalactosamine (Gal/GalNAc) polypeptide fragment, and preparing method and application of polypeptide fragment
An amoebic galactose, acetamidosemi technology, applied in DNA/RNA fragments, chemical instruments and methods, peptides, etc., can solve the problem of unclear role of intermediate subunits, large molecular weight of full-length Igl, prone to cross-reaction, etc. It can improve the specificity and sensitivity, and overcome the missed detection and misdiagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Culture of Entamoeba histolytica
[0044] Entamoeba histolytica HM-1: IMSS strain was aseptically cultured in BI-S-33 medium containing 15% adult bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin. Amoeba trophozoites were cultured in a 6ml glass culture tube with a cover, and the tube cap was tightly closed, and it was 5 o placed in a 36.5°C cell culture incubator.
Embodiment 2
[0045] Example 2 PCR reaction amplifies igl1 C-terminal gene
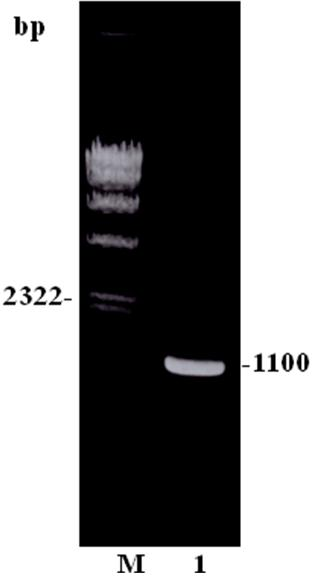
[0046] According to the kit instructions, use the RNeasy Total RNA kit to extract the total RNA of the aseptically cultured Entamoeba histolytica HM-1:IMSS strain. RT-PCR was performed using GeneAmp RNA PCR Kit to obtain cDNA. Using cDNA as a template, the C-terminal gene was amplified with primers EH150-S749-XHO (5’-CCCTCGAGACTGAACAAAGGCTAAAAGA-3’) and EH150-AS1088-XHO (5’-CCCTCGAGTTAAATGCCTTTAGCTCCATT-3). PCR execution program: 94°C 2min; 94°C 15sec, 55°C 30sec, 72°C 1min, a total of 30 cycles; 72°C 3min. After the reaction, each PCR product was subjected to 0.8% agarose gel electrophoresis. igl1 The C-terminal gene size is 1100bp (eg figure 1 shown).
Embodiment 3
[0047] Example 3 Construction of pET19b-Eh150-C plasmid
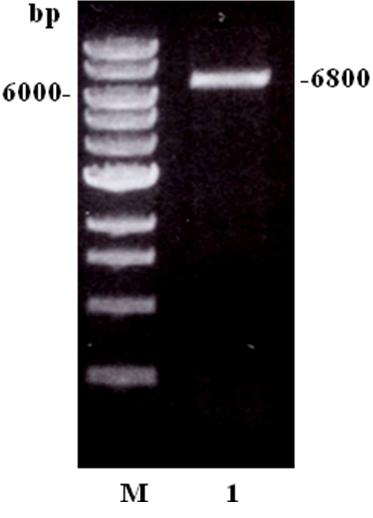
[0048] After purifying the PCR amplified product with QIAquick PCR Purification Kit, the PCR amplified product was digested with Xho I. After 0.8% agarose gel electrophoresis, DNA was purified with QIAEXII Agaose Gel Extraction Kit. The vector pET19b and the C-terminal gene fragment of the Igl gene were ligated with TAKARA DNA Ligation Kit overnight at 16°C. The ligation product was transformed into JM109 competent cells, cultured overnight at 37°C, and the plasmid was extracted. The results of 0.8% agarose gel electrophoresis showed that the recombinant gene fragments were successfully connected to the plasmid, and the size of the recombinant plasmid was 6.8kb, (such as figure 2 shown), sequencing identification confirmed that the insertion site of the target gene and the size of the insertion fragment were correct. Using Vector NTI 10 software, the amino acid sequence was deduced, and compared with the amino acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com