Deep-sea cold-adapted and salt-tolerant collagenase as well as encoding gene myr02 and application of same
A collagenase and cold-adaptive technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of less collagenase and limited application, and achieve good cold adaptability, salt tolerance, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]A strain of Myroides profundi D25, which was preserved in the China Center for Type Culture Collection on July 13, 2008, address: China, Wuhan, Wuhan University, strain preservation number: CCTCC NO.M 208030.
Embodiment 2
[0036] The nucleotide sequence of the collagenase gene myr02 extracted from Myroides profundi D25 is shown in SEQ ID NO.1.
[0037] The collagenase myr02 encoded by the above collagenase gene myr02 has an amino acid sequence shown in SEQ ID NO.12.
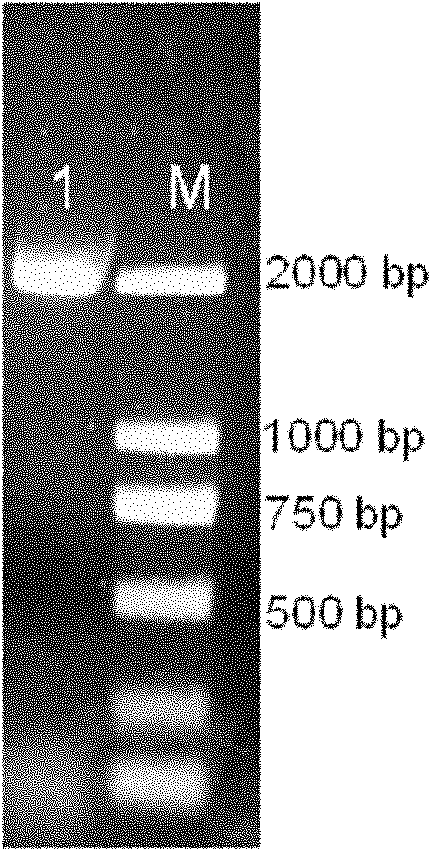
[0038] Collagenase gene myr02 contains a 2040bp open reading frame encoding collagenase myr02, the start codon is located at 1bp, the stop codon is located at 2038bp, and a total of 679 amino acids are encoded. The corresponding relationship between nucleotides and encoded amino acids in the gene is as follows: Figure 7 shown.
[0039] The preparation method of collagenase gene myr02 is as follows:
[0040] 1. Cloning of the gene encoding collagenase myr02
[0041] 1.1 For the extraction of the genomic DNA of Myroides profundi D25, refer to the instructions of the Genome Extraction Kit of Biotec Company:
[0042] (1) Take 1ml of Myroides profundi D25 bacteria liquid, centrifuge at 10000rpm for 30sec, discard the supernatant, co...
Embodiment 3
[0081] Example 3: Purification of collagenase myr02
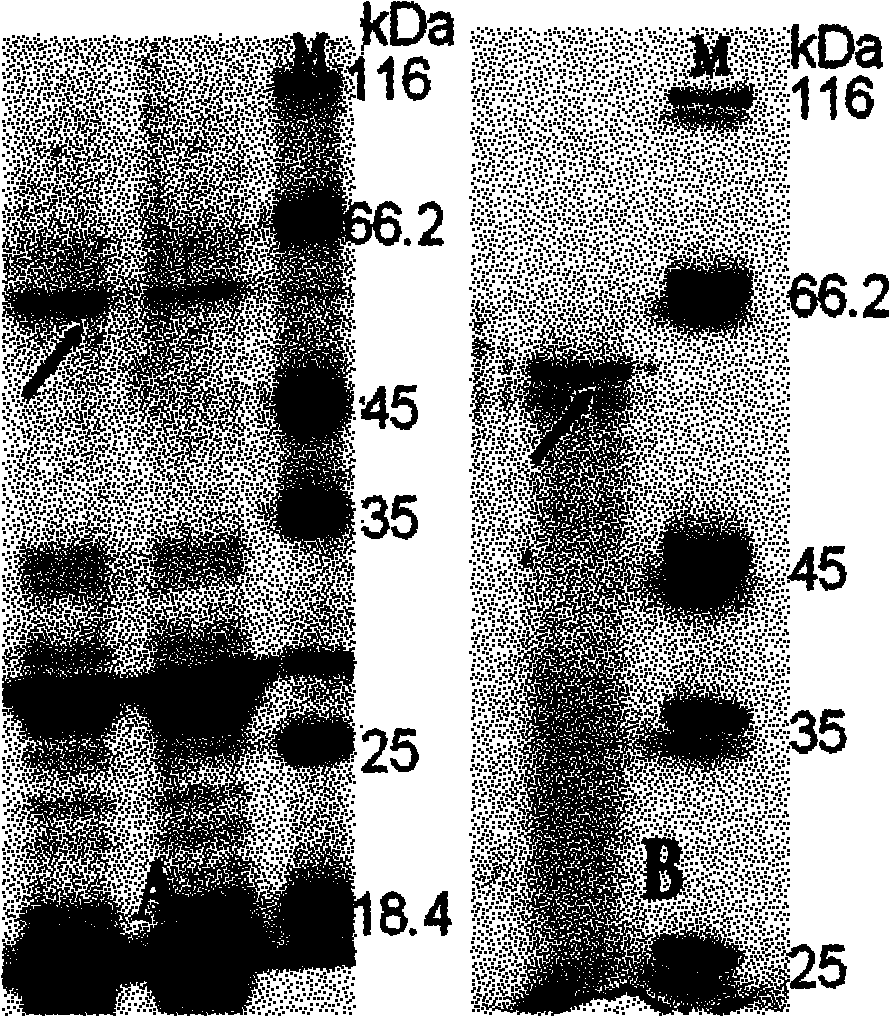
[0082] The psychrotrophic bacteria (Myroides profundi) D25 was inoculated in the fermentation medium (artificial seawater containing 0.2% yeast powder, 1% gelatin, pH 8.5), and cultured at 15° C., 200 rpm for 84 hours. The fermentation broth was centrifuged at 10000g for 10min at 4°C. The supernatant was collected for 55% ammonium sulfate precipitation, left standing overnight, 4°C, centrifuged at 10,000g for 10min to collect the precipitate, redissolved with 50mM Tris-HCl buffer (pH 9.5) and dialyzed overnight for desalination, then centrifuged at 10,000g for 15min at 4°C. The supernatant was passed through a DEAE-Sepharose Fast Flow chromatography column at a speed of 0.4ml / min and then eluted with a gradient of 0-0.8M NaCl. The eluted active fraction was collected, concentrated and further separated and purified by supherdex G75. Purified protease was tested for purity by 7.5% SDS-PAGE ( image 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com