Functional ionic liquid and preparation method thereof
An ionic liquid, functionalized technology, applied in chemical instruments and methods, preparation of sulfonic acid amides, organic chemistry, etc., can solve the problems of difficult coordination of metal ions and no lone pair electrons.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] ①. Dissolve 30g of phenanthroline in 150mL of concentrated sulfuric acid (65%-68%) and add 80mL of fuming nitric acid (86%-97.5%) dropwise under stirring at 168°C. Slowly pour the mixture into more than 2Kg of crushed ice to cool it below zero, then add NaOH to adjust the pH to about 7, filter and wash with water to obtain 5-nitro-1,10-phenanthroline, which is recorded as Phen-NO 2 (32.7782g).
[0038] ②, 1g Phen-NO 2 Dissolve in 10mL ethanol and add 200mg of activated carbon (5%Pd / C) loaded with 5% palladium metal powder by mass percentage, then mix 1.5mL hydrazine with 10mL ethanol, drop this at room temperature, then mix the solution at 70 Reflux under stirring at ℃ for 6-8 hours, filter and concentrate the filtrate to half of its original volume, then add 500mL of water and let stand until all 5-amino-1,10-phenanthroline is precipitated, filter and then purify in chloroform to obtain a yellow-green color The solid is put into a vacuum drying oven for drying and re...
Embodiment 2
[0049] The methylimidazole in step ④ was replaced with butylimidazole (commercially available), the ionic liquid was denoted as Phen-bmim (X=Cl, n=3, molecular weight=395.68), and the remaining conditions were the same as in Example 1.
[0050] The NMR of the tested ionic liquid is as follows:
[0051] 1 H NMR (DMSO): δ11.291(s, 1H), δ9.383(s, 1H), δ9.166(m, 1H), δ9.070(m, 1H), δ8.927(d, 1H) , δ8.487(m, 1H), δ8.203(s, 1H) δ7.915(s, 1H), δ7.876(m, 2H), δ7.771(m, 1H), δ5.585(s , 2H), δ4.291 (t, 2H), δ1.825 (t, 2H), δ1.310 (m, 2H), δ0.932 (s, 3H).
[0052] The physical and chemical parameters of the material tested are as follows:
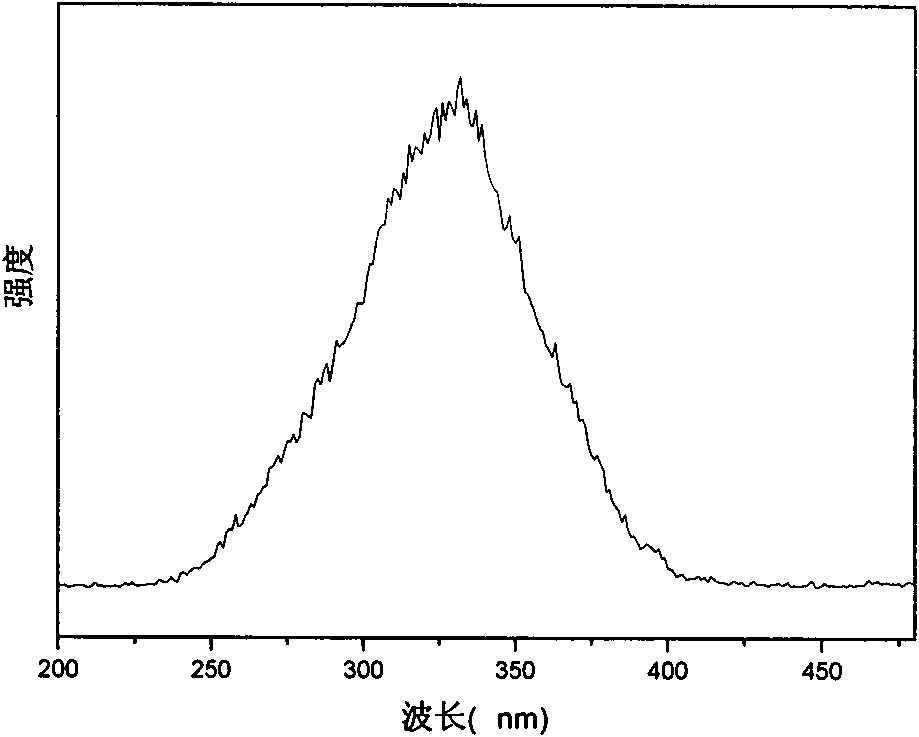
[0053] Excitation spectrum (detection wavelength: 612nm): 200-480nm
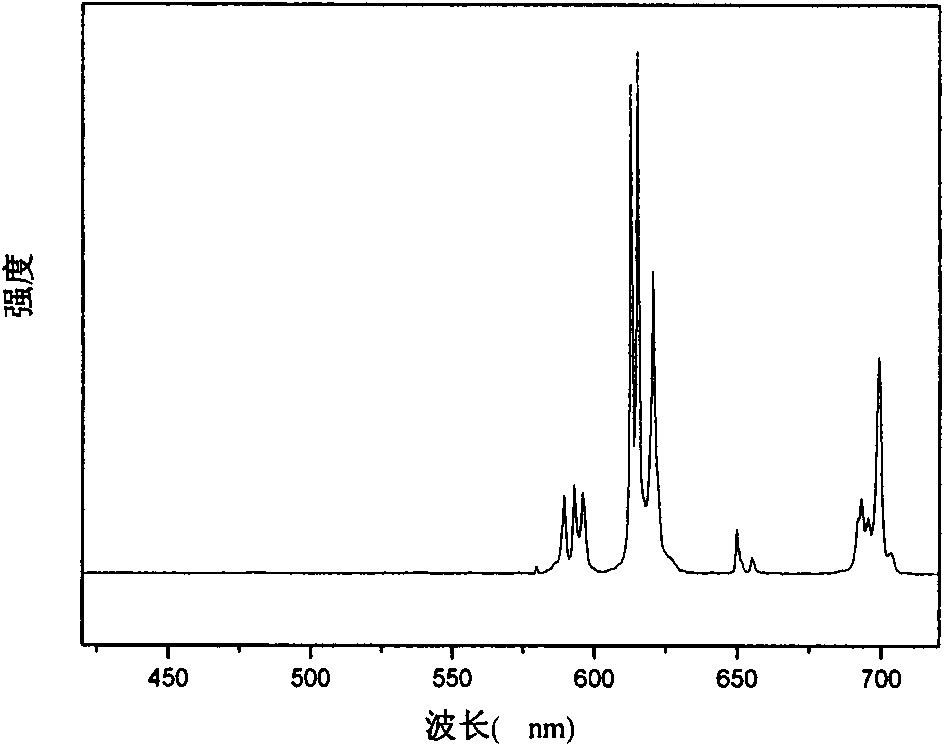
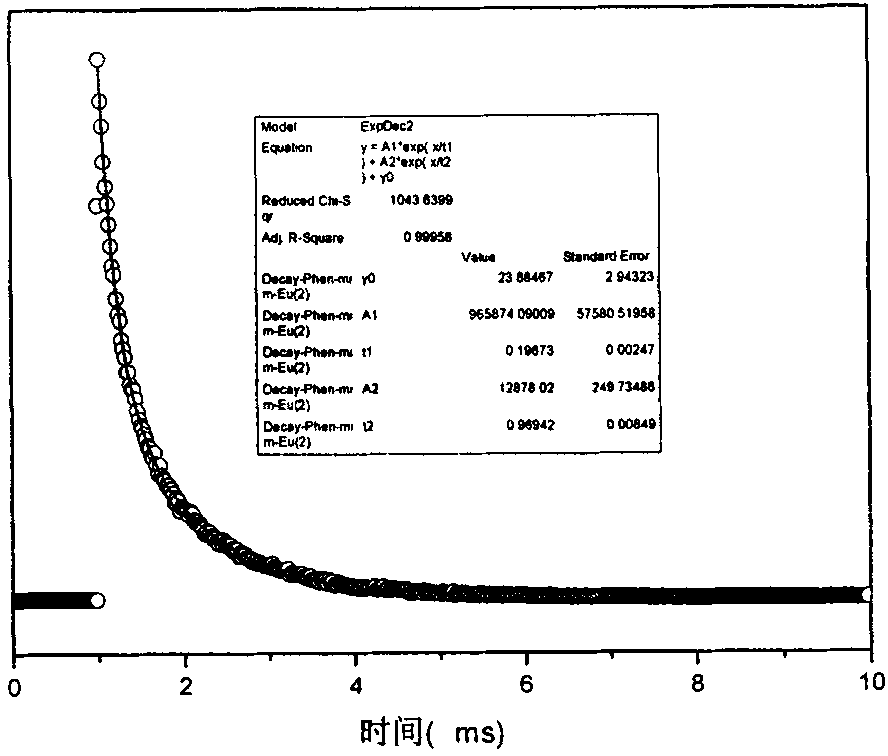
[0054] Figure 4 , 5, and 6 are the excitation, emission, and lifetime spectrograms in Example 2, respectively; the data of this rare earth / ionic liquid luminescent material are shown. The characteristic peak of europium can be well seen in the emission diagram, indicating tha...
Embodiment 3
[0056] Dissolve 10mmoL imidazole in 8mL tetrahydrofuran, 15mmoL sodium hydride in 4mL tetrahydrofuran, add the imidazole solution dropwise into the tetrahydrofuran solution of sodium hydride, a large number of bubbles are generated, stir at room temperature for 1 hour after the dropwise addition, add 0.3mmoL tetrabutylammonium iodide The system was evacuated and filled with nitrogen, and 10mmoL of bromoheptane dissolved in 6mL of tetrahydrofuran was added to the system and stirred at room temperature for 18 hours, then filtered with suction, and the filtrate was rotary evaporated, extracted with ether and water, and heptanimidazole was obtained.
[0057] The methylimidazole in step ④ in Example 1 was replaced by heptanimidazole, and the ionic liquid was denoted as Phen-ILC7 (X=Cl, n=6, molecular weight=437.77), and the rest of the conditions were the same as in Example 1.
[0058] The NMR of the tested ionic liquid is as follows:
[0059] 1 H NMR (CDCl 3 ): δ11.537(s, 1H), δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com