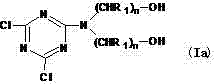Triazine compound coordination crosslinking synergistic agent for elastomer and preparation method thereof
A technology of coordination crosslinking and triazines, which is applied in the field of preparation of hydroxyalkane chain-substituted triazines, can solve problems such as unreported research, and achieve improved crosslinking efficiency, strong chemical reactivity, and good coordination crosslinking. synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 15mL of acetone to a 200mL four-neck flask equipped with a reflux device, a stirrer, a constant pressure dropping funnel and a thermometer, place the flask in an ice-salt bath, and control the temperature at 1°C to 3°C, then add 1.8450g (0 .010mol) cyanuric chloride, stir and disperse evenly, join 1.0514g (0.010mol) diethanolamine in the cyanuric chloride solution, stir, meanwhile, a small amount of sodium hydroxide solution (0.40gNaOH is dissolved in 10mL distilled water ), control the pH value of the reaction system between 7.0±0.2, and react for 3 hours to obtain the intermediate product Ia 1 . Intermediate Ia 1 It is 2-(N,N-dihydroxyethyl)amino-4,6-dichloro-1,3,5-triazine compound, the theoretical structural formula is as follows:
[0030]
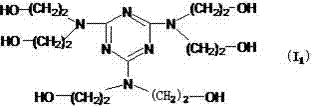
[0031] Intermediate Ia 1 Does not require separation and purification, to contain Ia 1 Add 15mL of water to the reaction solution, accelerate stirring evenly, then put 2.2079g (0.021mol) diethanolamine into the soluti...
Embodiment 2
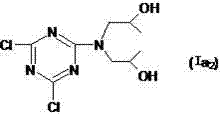
[0037] Add 15 mL of N,N-dimethylacetamide-acetone mixed solvent with a volume ratio of 1:1 to a 200 mL four-neck flask equipped with a reflux device, a stirrer, a constant pressure dropping funnel and a thermometer, and place the flask on ice In the brine bath, the temperature is controlled at -1°C ~ 1°C, then add 1.8450g (0.010mol) cyanuric chloride, stir and disperse evenly, add 1.4651g (0.011mol) diisopropanolamine to the cyanuric chloride solution, stirring, and at the same time, a small amount of potassium hydroxide solution (0.56gKOH dissolved in 10mL distilled water), control the pH value of the reaction system between 6.5±0.2, and react for 1.5 hours to obtain the intermediate product Ia 2 . Intermediate Ia 2 It is 2-[N,N-di(2-hydroxy)propyl]amino-4,6-dichloro-1,3,5-triazine compound, the theoretical structural formula is as follows:
[0038]
[0039]Intermediate Ia 2 Does not require separation and purification, to contain Ia 2 Add 20mL of water to the reaction...
Embodiment 3
[0045] Add 20mL of tetrahydrofuran to a 200mL four-necked flask equipped with a reflux device, a stirrer, a constant pressure dropping funnel and a thermometer, place the flask in an ice-salt water bath, and control the temperature at 0°C to 2°C, then add 1.8450g (0.010 mol) cyanuric chloride, stir and disperse evenly, join 3.1392g (0.011mol) phenyl diisopropanolamine in the cyanuric chloride solution, stir, simultaneously, a small amount of sodium carbonate solution (0.53gNa 2 CO 3 dissolved in 10mL distilled water), control the pH value of the reaction system between 6.5±0.2, and react for 2 hours to obtain the intermediate product Ia 3 . Intermediate Ia 3 It is 2-[N,N-bis(1-phenyl-2-hydroxy)propyl]amino-4,6-dichloro-1,3,5-triazine compound, the theoretical structural formula is as follows:
[0046]
[0047] Intermediate Ia 3 Does not require separation and purification, to contain Ia 3 Add 25mL of water to the reaction solution, accelerate the stirring evenly, then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com