Wholly-aromatic polymer fiber with high compound property and preparing method thereof
An aromatic polymer and composite technology, which is applied in fiber type, fiber treatment, textiles and papermaking, etc., can solve the problems of aramid fiber mechanical performance degradation and fracture, and achieve the suppression of fiber body mechanical performance degradation and fluorine gas consumption. Small size, good interface bonding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
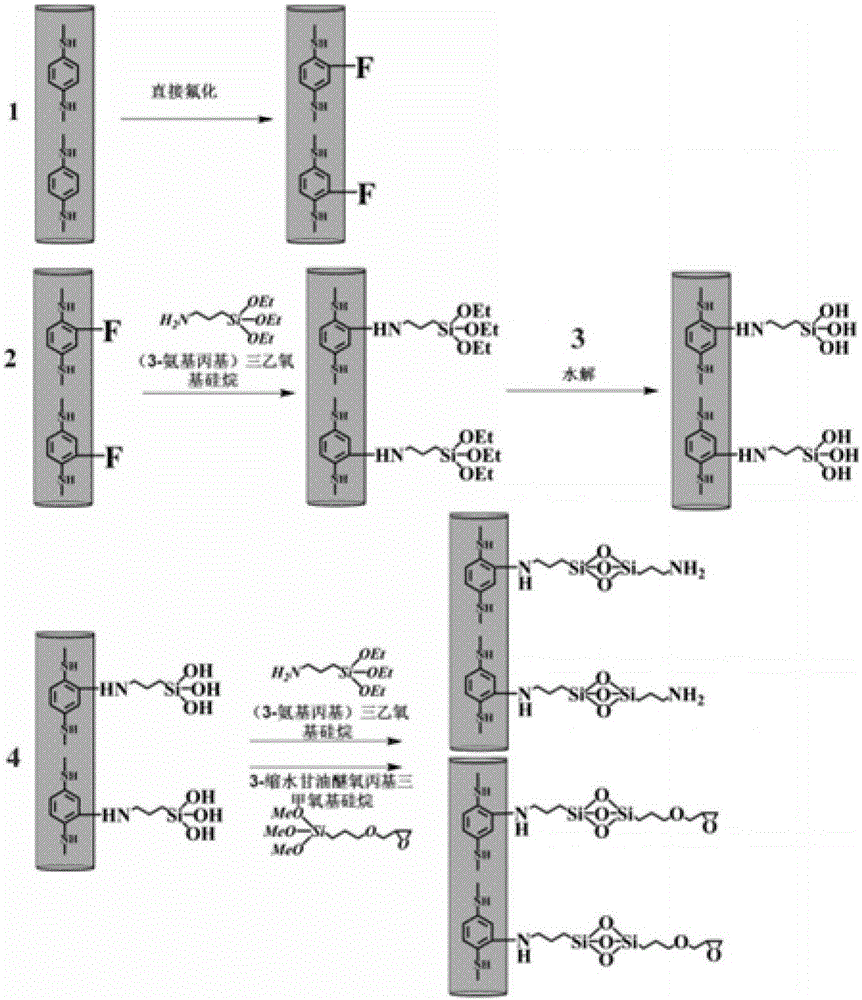
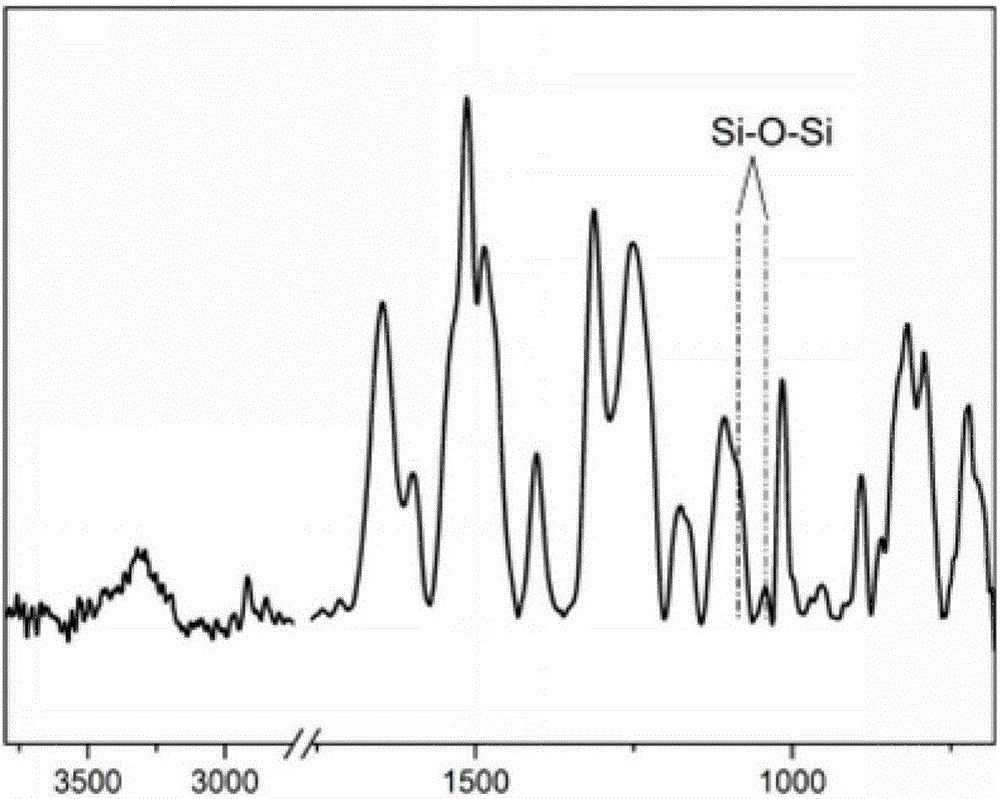
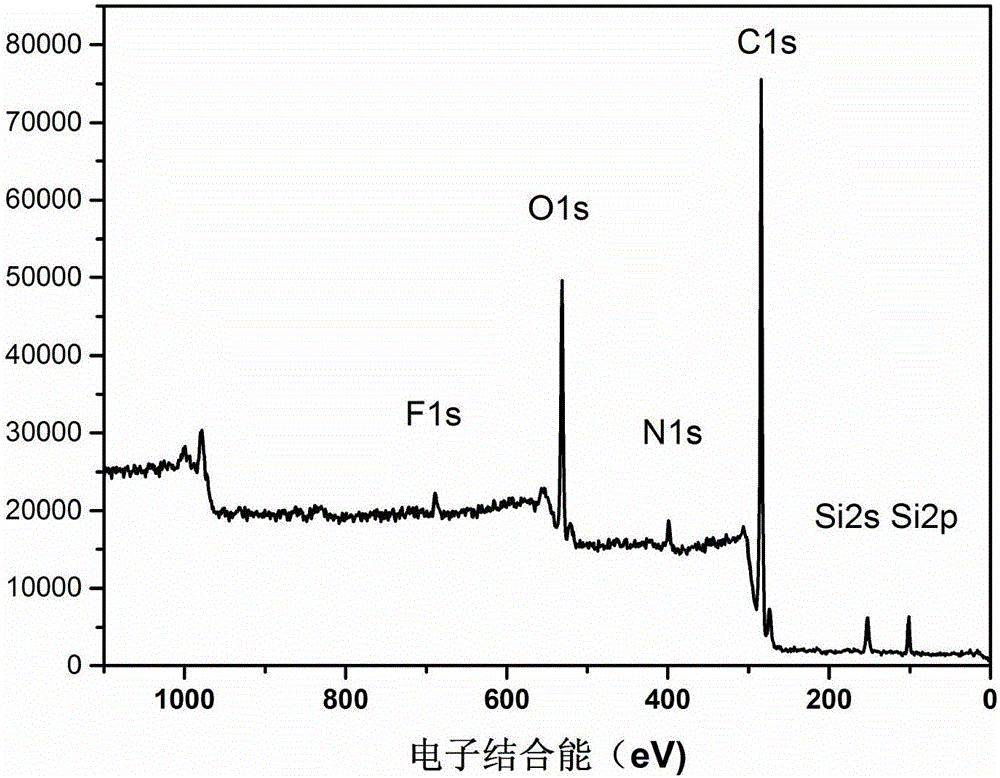
[0036] First, the aramid III fiber prepared according to the monomer molar ratio TPC:PDA:PABZ=10:5:5 is directly fluorinated under the fluorination partial pressure of 1kPa and the nitrogen partial pressure of 10kPa by the method disclosed in the prior art treatment, then statically soak the fluorinated fibers in an ethanol solution of 2% (3-aminopropyl) triethoxysilane to react for 30 minutes, then dry them, and then statically soak them in an aqueous hydrochloric acid solution with pH=4 Drying is carried out after 25 minutes of neutral hydrolysis reaction, and finally the fibers are put into ethanol solution with a concentration of 2% (3-aminopropyl) triethoxysilane, soaked and reacted for 30 minutes, and then dried.
[0037] The obtained high-composite performance aramid fiber III is added to the epoxy resin matrix to prepare the corresponding composite material, and the relevant properties of the obtained composite material are shown in the attached table.
Embodiment 2
[0039] First, the aramid III fiber prepared by the monomer molar ratio TPC:PDA:PABZ=10:5:5 is directly fluorinated under the fluorination partial pressure of 0.5kPa and the nitrogen partial pressure of 5kPa by the method disclosed in the prior art. fluorination treatment, and then statically soak the fluorinated fibers in an ethanol solution of 2% (3-aminopropyl) triethoxysilane to react for 30 minutes, then dry them, and then statically soak them in hydrochloric acid with pH=4 After 30 minutes of hydrolysis reaction in the aqueous solution, drying is carried out, and finally the fiber is put into an ethanol solution with a concentration of 3% (3-aminopropyl) triethoxysilane, soaked and reacted for 30 minutes, and then dried.
[0040] The obtained high-composite performance aramid fiber III is added to the epoxy resin matrix to prepare the corresponding composite material, and the relevant properties of the obtained composite material are shown in the attached table.
Embodiment 3
[0042] First, the aramid III fiber prepared according to the monomer molar ratio TPC:PDA:PABZ=10:5:5 is directly fluorinated under the fluorination partial pressure of 1.5kPa and the nitrogen partial pressure of 15kPa by the method disclosed in the prior art. fluorination treatment, then statically immerse the fluorinated fibers in an ethanol solution of 1% (3-aminopropyl) trimethoxysilane to react for 20 minutes, then dry them, and then statically immerse them in an aqueous solution of sulfuric acid with pH=5 Drying is carried out after 20 minutes of neutral hydrolysis reaction, and finally the fiber is put into an ethanol solution with a concentration of 2% 3-glycidyl etheroxypropyltrimethoxysilane, soaked and reacted for 40 minutes, and then dried.
[0043] The obtained high-composite performance aramid fiber III is added to the epoxy resin matrix to prepare the corresponding composite material, and the relevant properties of the obtained composite material are shown in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com