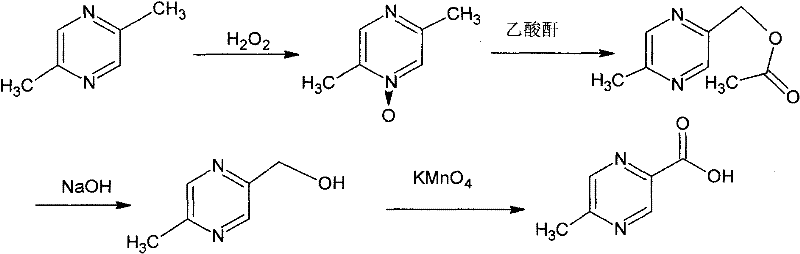
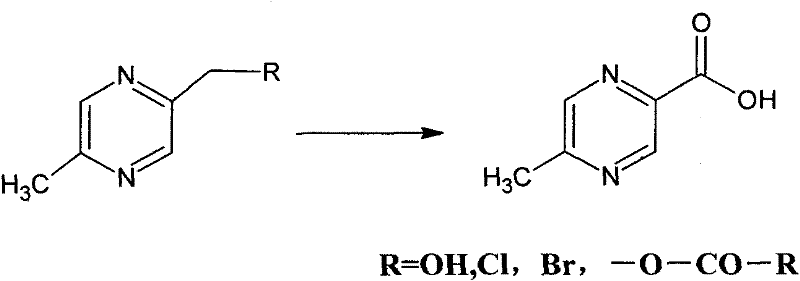
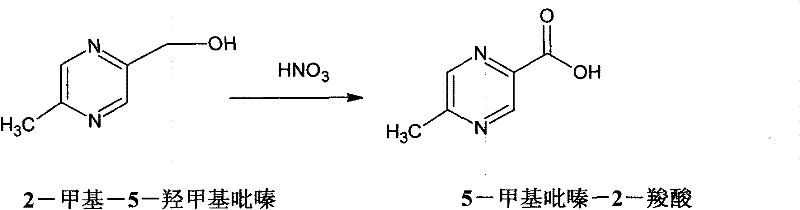
Method for synthesizing 5-methylpyrazine-2-carboxylic acid
A technology of methylpyrazine and hydroxymethylpyrazine, applied in the direction of organic chemistry, etc., can solve problems such as the difficulty in industrial design of electrochemical methods, and achieve the effects of low cost, high yield, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 carries out following processing step in sequence:
[0045]Add 12.4g of 2-methyl-5-hydroxymethylpyrazine (0.1mol), 50ml of water, and 0.15g of ammonium metavanadate to a 250ml three-necked flask, raise the temperature to 100°C, add 29g (0.3mol) of 65% nitric acid dropwise, for 1h After dripping, keep reflux for 4 hours, cool to room temperature; neutralize with 30% sodium hydroxide aqueous solution to pH = 2.0, distill off about 50g of water under reduced pressure, and extract with butanone three times, each time using 50ml of butanone, Then, butanone was distilled off to obtain 8.28 g of 5-methylpyrazine-2-carboxylic acid, the liquid chromatography analysis was 99.6%, and the yield was 60%.
Embodiment 2
[0046] Embodiment 2 carries out following processing steps in sequence:
[0047] Add 12.4g 2-methyl-5-hydroxymethylpyrazine (0.2mol) to a 250ml three-necked flask, 50ml water, 0.15g ammonium metavanadate, heat up to 100°C, add 38.8g (0.4mol) of 65% nitric acid dropwise, After 1 hour of dripping, keep reflux for 4 hours to cool to room temperature; use 30% potassium hydroxide aqueous solution to neutralize to pH = 1.8, evaporate about 50g of water under reduced pressure, and extract three times with cyclohexanone, the amount of cyclohexanone used each time The aqueous phase was extracted into 50 ml, and then the cyclohexanone was distilled off to obtain 8.97 g of 5-methylpyrazine-2-carboxylic acid. The liquid chromatography analysis was 99.5%, and the yield was 65%.
Embodiment 3
[0048] Embodiment 3 carries out following processing step in sequence:
[0049] Add 12.4g 2-methyl-5-hydroxymethylpyrazine (0.1mol) to a 250ml three-necked flask, 50ml water, 0.15g sodium metavanadate, heat up to 100°C, add 58.2g (0.6mol) of 65% nitric acid dropwise, After 1 hour of dripping, keep reflux for 8 hours; cool to room temperature, neutralize to PH=1.5 with saturated aqueous sodium carbonate solution, evaporate about 50g of water under reduced pressure, extract the water phase with 50ml×3 ethyl acetate, and then evaporate Ethyl acetate was used to obtain 10.76 g of 5-methylpyrazine-2-carboxylic acid, the liquid chromatography analysis was 99.4%, and the yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com