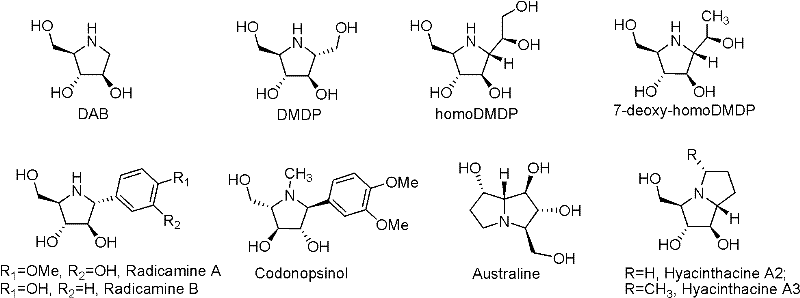
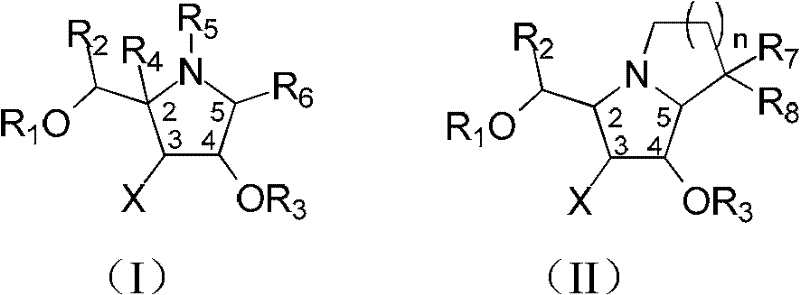
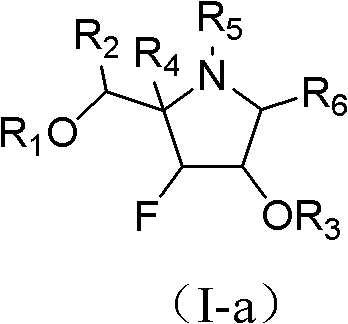
Fluoro imido sugar compounds, and preparation method and application thereof
A technology of fluorinated iminosugar and substituted iminosugar, which is applied in the field of fluoroiminosugar compound and its preparation and application, and can solve the problems of poor selectivity, lengthy steps, low efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation of intermediate IV-1 is carried out with reference to literature [(a) L.S.Jeong, B.B.Lim and V.E.Marquez, Carbohyd.Res., 1994,262,103-114; (b) T.Yoshiaki, U.Chiga, T. .Tsutomu and K.Yoshihiko, Carbohyd.Res., 1993,249,57-76.], the specific preparation method is: diacetone 3-deoxy-3-fluoroglucose (10.3g, 39.3mmol) is dissolved in 50mL tetrahydrofuran 5 mL of 1N hydrochloric acid was added, and stirred overnight at room temperature. Add 1N sodium hydroxide solution to neutralize the acid in the system until the pH value is about 8, evaporate the solvent and water, wash the residue with ethyl acetate and ethanol, discard the insoluble matter, and evaporate the organic phase to dryness to obtain the 5 , a monopropylidene intermediate of 6-propylidene, which is directly used in the next reaction.
[0059] The monopropylidene intermediate obtained in the previous step (calculated by 39.3mmol) was dissolved in 50mL of methanol and 10mL of water, and sodium peri...
Embodiment 1
[0081] Embodiment 1, the preparation of fluoroimino sugar formula (I-1), formula (I-2) and formula (I-3)
[0082] Taking the preparation of fluoroimino sugar formula (I-1), formula (I-2) and formula (I-3) as an example, the synthesis of such fluoroimino from fluoronitrone formula (III-1a) is illustrated Sugar step.
[0083]
[0084] Dissolve fluoronitrone formula (III-1a) (4.0g, 12.2mmol) in 30mL tetrahydrofuran, under nitrogen protection, cool to 0°C, add dropwise 20mL tetrahydrofuran solution of vinylmagnesium chloride (16M, 32.0mmol), reaction 5 Minutes later, it was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, and the organic phase was dried, evaporated to dryness and directly used in the next step.
[0085] Add copper acetate (0.3g, 1.5mmol), reduced iron powder (7.9g, 0.14mol) and 25mL glacial acetic acid into a 100mL flask, and stir for 1 hour until the blue color of copper ions disappears. Add the acetic acid solut...
Embodiment 2
[0093] Embodiment 2, preparation of fluoroimino sugar formula (I-4) and formula (I-5)
[0094] Taking the preparation of fluoroimino sugar formula (I-4) and formula (I-5) as an example, the synthesis of 3-deoxy-3-fluoro LAB from fluorine-containing intermediate XI-1 and its N-alkylation are illustrated product steps.
[0095]
[0096] Intermediate XI-1 (0.2g, 0.6mmol) was dissolved in 10mL of methanol, 40mg of palladium carbon (10%) and 10mL of 6N hydrochloric acid were added, and reacted under hydrogen atmosphere for 72 hours, the palladium carbon was recovered by filtration, and the filtrate was evaporated to dryness. Add concentrated ammonia water to neutralize the remaining acid, evaporate to dryness under reduced pressure again, and separate and purify with strong acidic ion exchange resin to obtain the product formula (I-4), which is dark yellow syrup, 76.6 mg, yield: 89%. δ H (300MHz; D 2 O) 4.67 (1H, d, J = 52.2Hz, H4), 4.27 (1H, ddd, J = 15.9Hz, 2.7Hz, 2.4Hz, H3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com