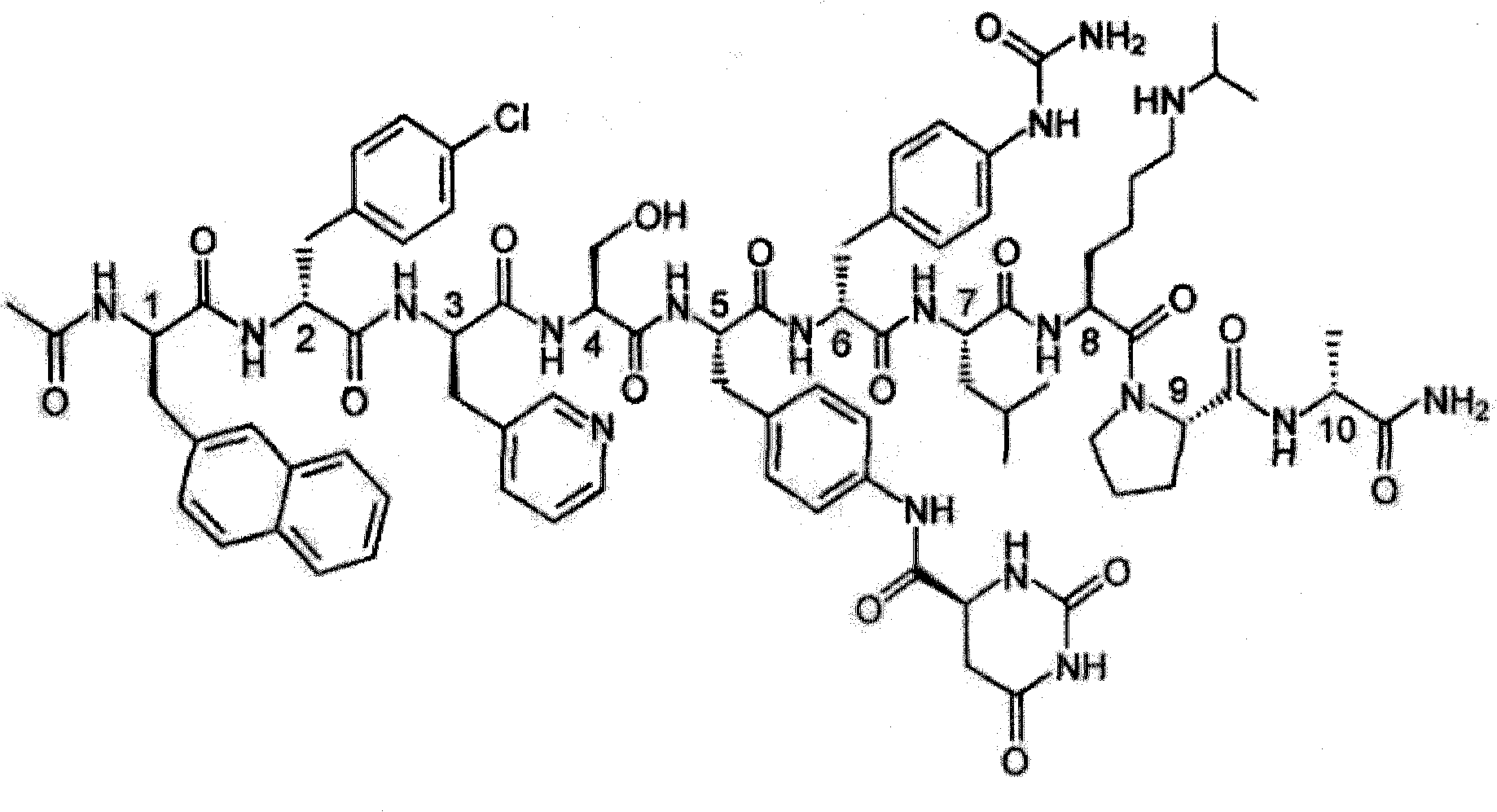
Degarelix acetate lyophilized powder injection and preparation method thereof
A technology of degarelix acetate and freeze-dried powder for injection is applied in the field of degarelix acetate freeze-dried powder for injection and its preparation, which can solve problems such as instability, achieve convenient clinical use, low production cost, and repeatability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] Accurately weigh mannitol and sodium acetate, add 90% water for injection, dissolve and stir evenly, then add degarelix acetate, dissolve and stir evenly, adjust pH to 5.0, after passing the intermediate inspection, add water for injection to the full amount. The solution is pumped into the sterile room, filtered through a 0.22 μm microporous membrane until it is clear, filled into 10 ml vials with a volume of 4 ml per bottle, added with a butyl rubber stopper, and placed in a plate. Put the plated sample to be freeze-dried in the freeze-drying oven, close the door, turn on the machine, and cool down. When the temperature of the product reaches -40°C, keep it for 3 hours, and then open the condenser valve. When the temperature of the condenser valve reaches -45°C At ℃, turn on the vacuum pump. When the vacuum reaches below 20Pa, start to heat up and sublimate and dry. The final drying temperature is 25°C. Keep this temperature for 2 hours. , you can.
Embodiment 2
[0021]
[0022] Accurately weigh mannitol and sodium acetate, add 90% water for injection, dissolve and stir evenly, then add degarelix acetate, dissolve and stir evenly, adjust pH to 7.4. After passing the intermediate inspection, add water for injection to the full amount. The solution is pumped into the sterile room, filtered through a 0.22 μm microporous membrane until it is clear, filled into 10 ml vials with a volume of 4 ml per bottle, added with a butyl rubber stopper, and placed in a plate. Place the plated samples to be freeze-dried in the freeze-drying oven, close the door, turn on the machine, and cool down. When the temperature of the product reaches -40°C, keep it for 2 hours, and then open the condenser valve. When the temperature of the condenser valve reaches -45°C At ℃, turn on the vacuum pump. When the vacuum reaches below 20Pa, start to heat up and sublimate and dry. The final drying temperature is 25°C. Keep this temperature for 4 hours. , you can.
Embodiment 3
[0024]
[0025]
[0026] Accurately weigh mannitol and sodium acetate, add 90% water for injection, dissolve and stir evenly, then add degarelix acetate, dissolve and stir evenly, adjust pH to 6.5. After passing the intermediate inspection, add water for injection to the full amount. The solution is pumped into the sterile room, filtered through a 0.22 μm microporous membrane until it is clear, filled into 10 ml vials with a volume of 4 ml per bottle, added with a butyl rubber stopper, and placed in a plate. Put the plated sample to be freeze-dried in the freeze-drying oven, close the door, turn on the machine, and cool down. When the temperature of the product reaches -40°C, keep it for 3 hours, and then open the condenser valve. When the temperature of the condenser valve reaches -45°C At ℃, turn on the vacuum pump. When the vacuum reaches below 20Pa, start to heat up and sublimate and dry. The final drying temperature is 25°C. Keep this temperature for 3 hours. , you...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com