Method for preparing diaryl carbonates and polycarbonates
A technology of diaryl carbonate and diphenyl carbonate, which is applied in the field of preparing diaryl carbonate and polycarbonate, and can solve problems such as low current output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a-h
[0204] Preparation of Diphenyl Carbonate by Direct Phosgenation of Phenol
[0205] 1a) In γ-Al 2 O 3 Preparation of diphenyl carbonate by phosgenation of phenol in the presence of
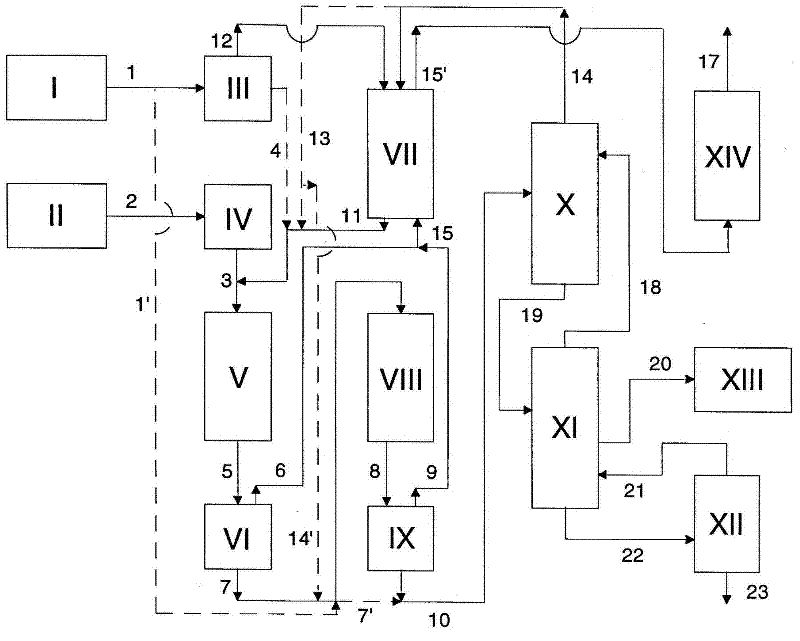
[0206] image 3 The apparatus used to carry out the method and the flows involved are schematically shown in .
[0207] 41.12 parts by weight / h of phenol 1 , fresh and / or recovered from the preparation of polycarbonate as described in example 3), were taken from heated vessel I and passed through heat exchanger III (60° C.) at Atmospheric pressure is fed from the top into a countercurrent packed column VII heated to 60° C., in which mixing with the phenol 14 withdrawn from the top of the distillation column X takes place. After the offgas stream 15 from the degassers VI and IX has passed through VII, the mixture 11 (phenol / phosgene weight ratio about 97 / 3) is fed in at the bottom. From this storage tank II, 21.93 parts by weight / h of phosgene 3 (170 °C) preheated by heat exchanger IV ...
Embodiment 2
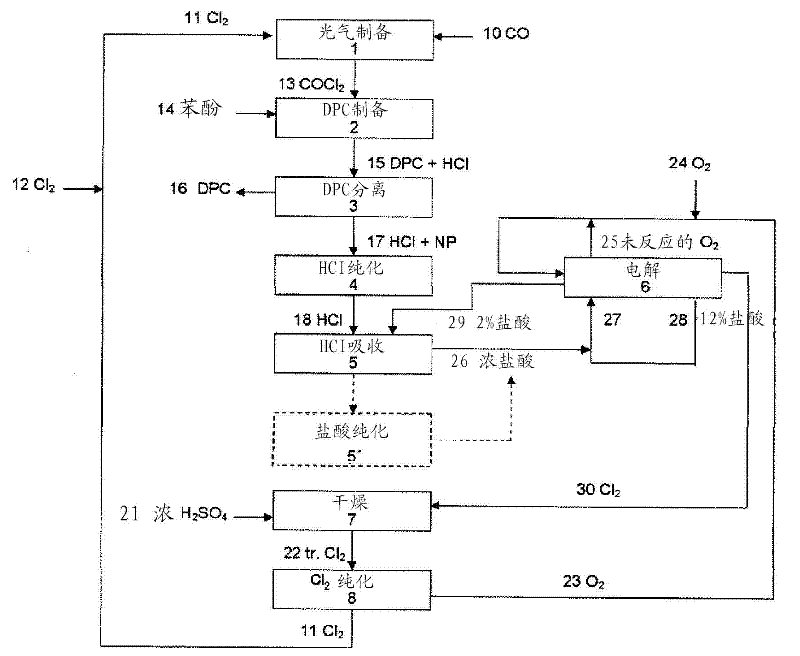
[0240] Hydrogen chloride produced from diphenyl carbonate is recycled by electrochemical oxidation with oxygen
[0241] a) Electrochemical oxidation of hydrogen chloride using a gas diffusion electrode as an oxygen-consuming cathode
[0242] A stream of 8.16 parts by weight / h of purified hydrogen chloride from the diphenyl carbonate production was fed to the HCl absorption. For this purpose, a substream of the hydrochloric acid-depleted anolyte acid stream 28 from the electrolysis is passed into the hydrochloric acid 26 from the HCl absorption. 32.1 parts by weight / h of the anolyte acid 28 , which is depleted in hydrochloric acid and has an HCl concentration of 12.2 wt %, is fed into the HCl absorption. The HCl absorption unit produces hydrochloric acid 26 at a concentration of 30 wt%, which is combined with the remainder of the lean anolyte acid 28 and returned to the electrolytic cell. 2.96 parts by weight / h of the lean anolyte acid 28 was withdrawn from the anolyte acid...
Embodiment 3
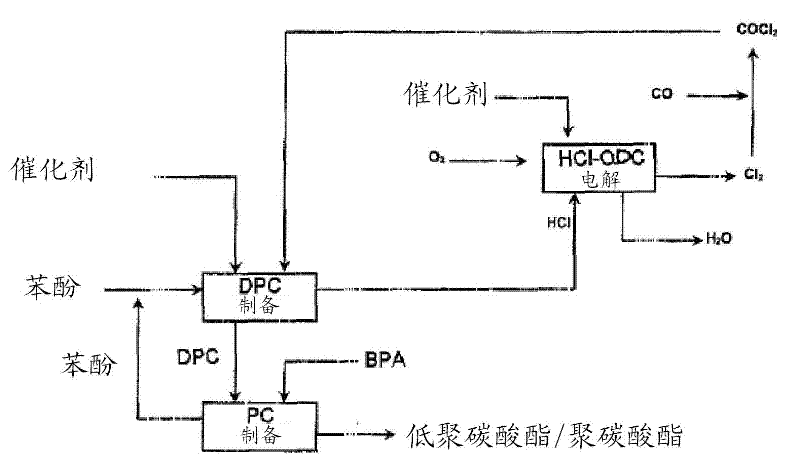
[0246] Preparation of polycarbonate
[0247]From a storage tank, a molten mixture of 8600 parts by weight / h containing 4425 parts by weight / h of diphenyl carbonate prepared as described in 1a) or 1b) and 4175 parts by weight / h of bisphenol A (and Add 0.52 parts by weight / h of tetraphenylphosphorus Phenol adduct of phenate comprising 65.5% tetraphenylphosphine dissolved in 4.5 parts by weight / h of phenol / h phenate / h) is pumped through a heat exchanger, heated to 190°C and sent through a retention column at 12 bar and 190°C. The average residence time was 50 minutes.
[0248] The melt was then introduced via a pressure relief valve into a separator at 200 mbar. The outgoing melt was reheated to 189° C. in a falling-film evaporator likewise at 200 mbar and collected in a vessel. After a residence time of 20 minutes, the melt was pumped into the following three stages of similar structure. The conditions in the second / third / fourth stages were 100 / 74 / 40 mbar, 218 / 251 / 276°C a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com