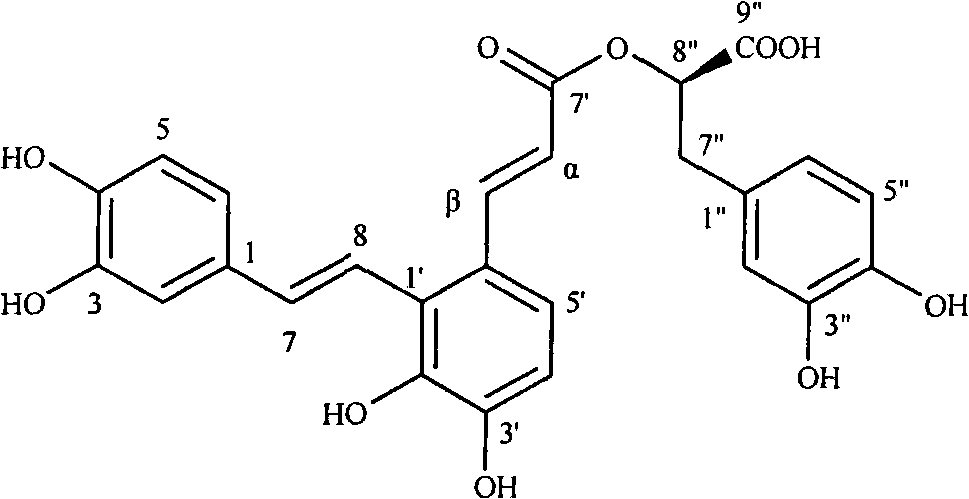
Medical use of salvianolic acid A
A technology of salvianolic acid and its use, which is applied in the new field of medical application, and can solve the problems such as the increase of cAMP content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
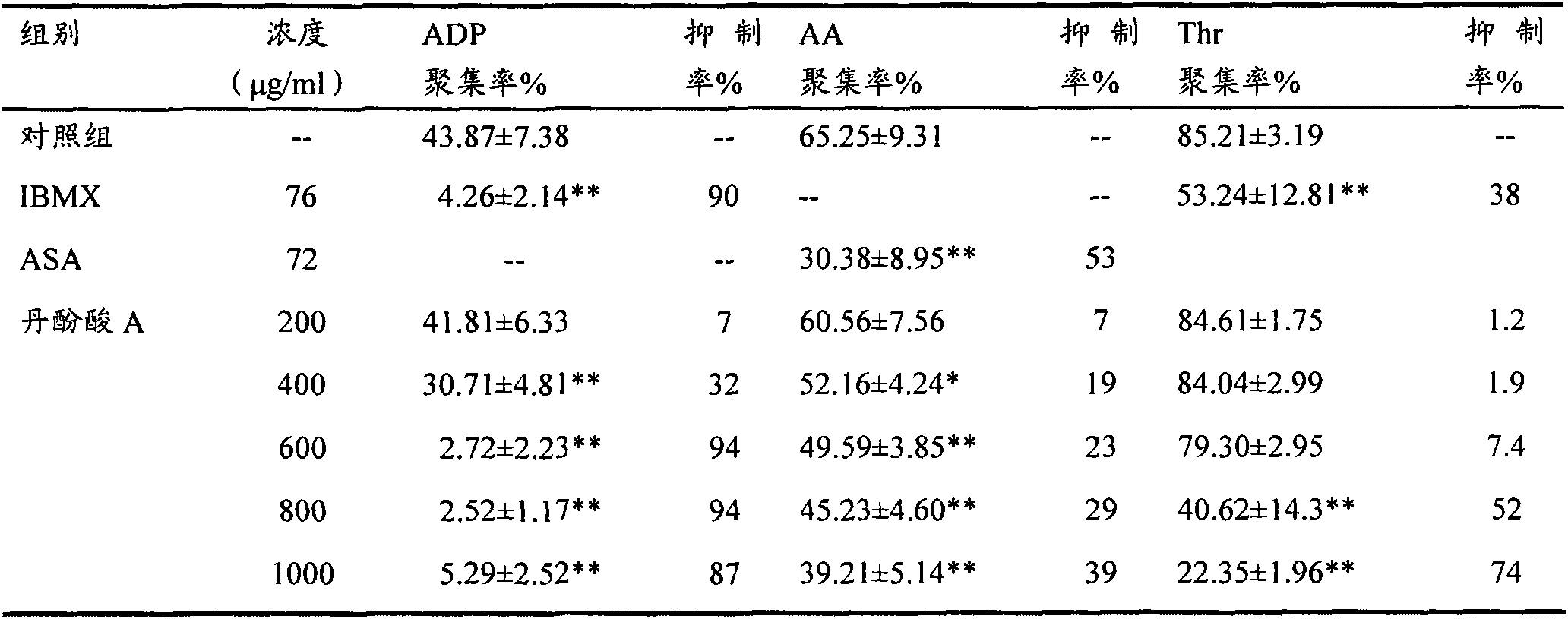
[0017] Experimental example 1: The effect of salvianolic acid A on platelet aggregation in vitro with different inducers
[0018] 1. Material
[0019] Salvianolic acid A, provided by Shandong Target Drug Research Co., Ltd.
[0020] Acetylsalicylic acid (ASA), sigma company A5376
[0021] 3-isobutyl-1-methylxanthine (IBMX), sigma company I5879
[0022] Adenosine diphosphate (ADP), sigma company A2754
[0023] Thrombin (Thr), sigma company T4648
[0024] Arachidonic acid (AA), sigma company BMA001
[0025] Ethylenediamine diacetic acid disodium salt (EDTA.Na 2 ), Tianjin No. 1 Chemical Reagent Factory, batch number: 080616
[0026] Ethylene glycol diethyl ether diamine tetraacetic acid (EGTA), sigma company E4378
[0027] Hepes, sigma company H3375
[0028] Bovine serum albumin fifth component (BSA), Roche 738328
[0029] Sodium Citrate, Sinopharm Chemical Reagent Co., Ltd., batch number: F20071212
[0030] Anhydrous calcium chloride (CaCl 2 ), Sinopharm Chemical Reagent Co., Ltd., batch number: ...
experiment example 2
[0050] Experimental example 2: The effect of salvianolic acid A on the content of cAMP in platelets and the activities of phosphodiesterase and adenylate cyclase in platelets
[0051] 1. The effect of salvianolic acid A on cAMP content in platelets
[0052] 1.1 Material
[0053] Salvianolic acid A, provided by Shandong Target Drug Research Co., Ltd.
[0054] 3-Isobutyl-1-methylxanthine (IBMX), sigma company, batch number: I5879, dissolved in physiological saline in a water bath at 100°C, with a concentration of 10mM.
[0055] Adenosine diphosphate (ADP), Sigma company, batch number: A2754, prepared with physiological saline to prepare a solution with a concentration of 300 μmol / l.
[0056] Ethylenediaminetetraacetic acid disodium salt (EDTA), Tianjin No. 1 Chemical Reagent Factory, batch number: 080616, prepared with deionized water.
[0057] Ethylene glycol diethyl ether diamine tetraacetic acid (EGTA), sigma company, batch number: E4378
[0058] Hepes, sigma company, batch number: H3375 ...
experiment example 3
[0104] Experimental Example 3: Determination of Salvianolic Acid A on Free [Ca2+]i in Platelets
[0105] 1. Material
[0106] Salvianolic acid A, provided by Shandong Target Drug Research Co., Ltd.
[0107] Adenosine Diphosphate (ADP): sigma company A2754
[0108] Acetyl hydroxymethyl ester (Fura-2 / AM): product of sigma
[0109] TritonX-100, purchased from Huamei Bioengineering Company
[0110] Animals: SPF-grade SD rats, male, weighing 300-350g, purchased from the Laboratory Animal Center of Peking University Medical Department, certificate number: SCXK (Beijing) 2006-0008.
[0111] 2. Methods and results
[0112] Blood was taken from the abdominal aorta of the rat, and 3.8% sodium citrate (1:9) was used for anticoagulation to prepare PRP. Take PRP, add Fura-2 / AM with a final concentration of 2μmol / L, shake for 30min at 37°C in the dark. Ca-free 2+ Wash 2 times with Tyrode-Hepes solution to remove excess Fura-2 / AM from platelets, and finally suspend in Ca 2+ Tyrode-Hepes solution, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com