Compound methoxyphenamine sustained release preparation
A technology for methoxyphenamine and sustained-release preparations, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pill delivery, etc. Methoxyphenamine products do not have problems such as sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
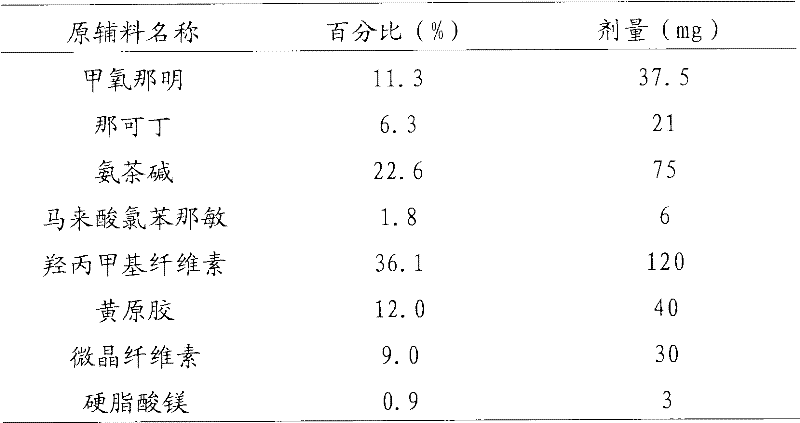
[0093] Example 1: Compound tablet with sustained release up to 12 hours
[0094]
[0095] Preparation Process:
[0096] According to the proportion of the above formula, the active ingredients (methoxamine, noscapine, aminophylline, chlorpheniramine maleate) are mixed with hydroxypropyl methylcellulose and xanthan gum and stirred evenly; add an appropriate amount of The ethanol solution is stirred into fine particles and dried; pulverized to make them all pass through the No. 2 sieve of the Chinese Pharmacopoeia; the pulverized particles are added with filler microcrystalline cellulose and lubricant magnesium stearate, and compressed into tablets;
Embodiment 2
[0097] Example 2: Compound tablet with sustained release up to 24 hours
[0098]
[0099]
[0100] Preparation Process:
[0101] According to the ratio of the above-mentioned formula amount, the active ingredients (methoxyphenamine, noscapine, aminophylline, chlorpheniramine maleate) are mixed with hydroxypropyl methylcellulose and sodium alginate and stirred evenly; add an appropriate amount of The ethanol solution is stirred into fine particles and dried; pulverized to make them all pass through the No. 2 sieve of the Chinese Pharmacopoeia; the pulverized particles are added with filler microcrystalline cellulose and lubricant magnesium stearate, and compressed into tablets;
Embodiment 3
[0102] Example 3: Compound capsules with sustained release up to 12 hours
[0103]
[0104] Preparation Process:
[0105] The active ingredients (Methoxamine, Noscapine, Aminophylline, Chlorpheniramine Maleate), polyethylene glycol, sodium alginate and filler microcrystalline cellulose are mixed and stirred according to the above formula amounts Evenly; add an appropriate amount of aqueous solution, use an extrusion spheronizer to form pellets with a diameter of about 1.0mm, dry them, and put them into capsules;
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com