Purification method for recombinant human interferon beta-1a
A technology of recombinant human interferon and purification method, which is applied in the field of new purification technology of recombinant human interferon beta-1a to achieve the effect of high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Expression of recombinant human IFNβ in mammalian cells
[0042] 1) Construction of cell lines
[0043] We optimized the human IFNβ gene sequence in Genbank by adopting mammalian biased codons, and artificially synthesized the IFNβ gene sequence (Sequence No.2) while keeping the encoded amino acid sequence (Sequence No.1) unchanged. ). Insert this artificially synthesized gene fragment into a high-efficiency eukaryotic expression plasmid vector pCG-IFNβ constructed by us, transfect CHO-K1 cells, and use methionyl sulfate to screen and establish a stably transfected cell line CHO-K1- 9A3, the expression of the protein was detected by immunoblotting and ELISA, and a cell line capable of secreting and expressing the protein at a high level was obtained. Then through the acclimatization of serum-free culture, a suspension growth cell strain suitable for serum-free culture was obtained.
[0044] 2) Identification of recombinant human IFNβ-1a cell line
[0045] ...
Embodiment 2
[0050] Purification route A of embodiment 2 recombinant human IFNβ-1a protein
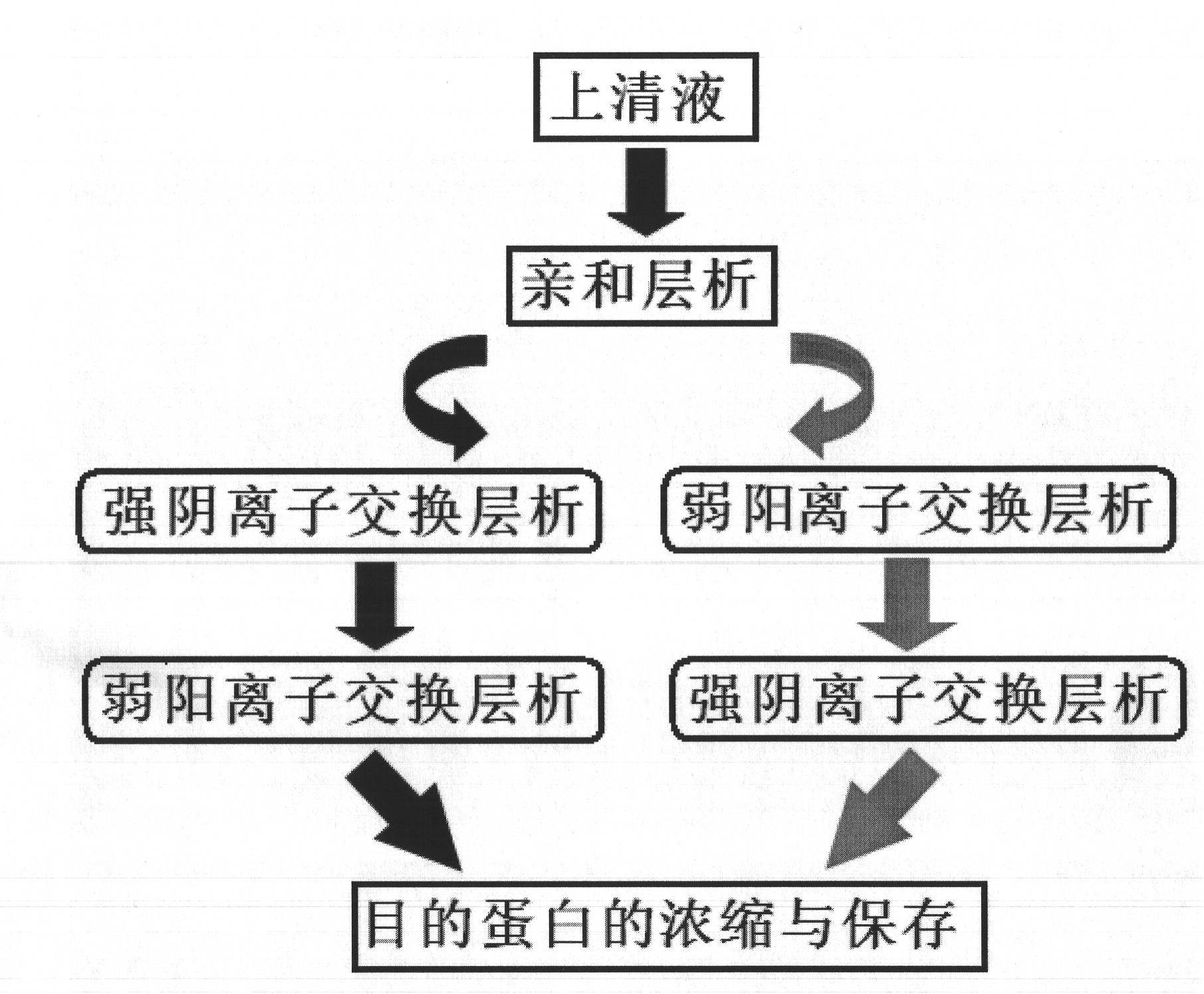
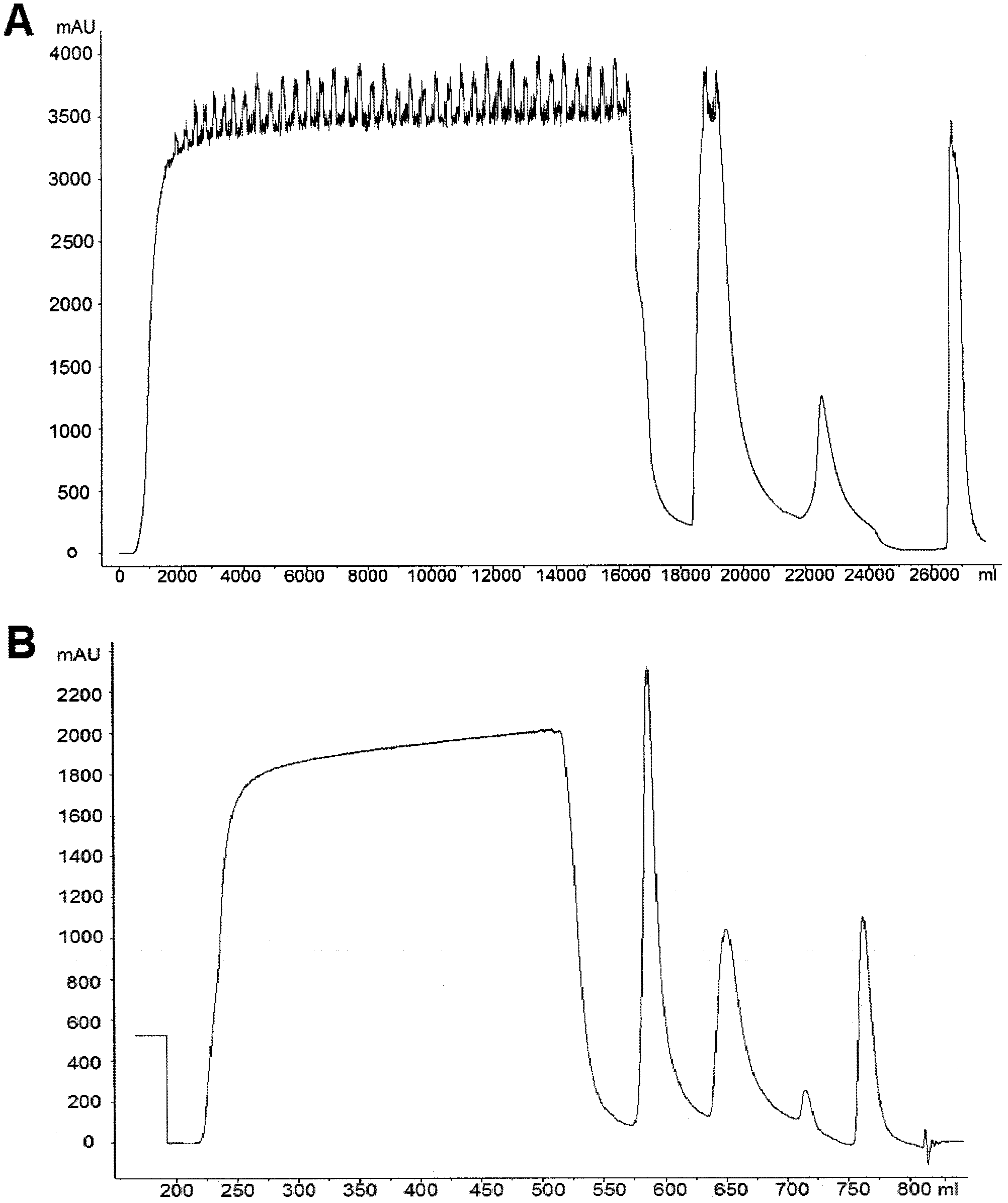
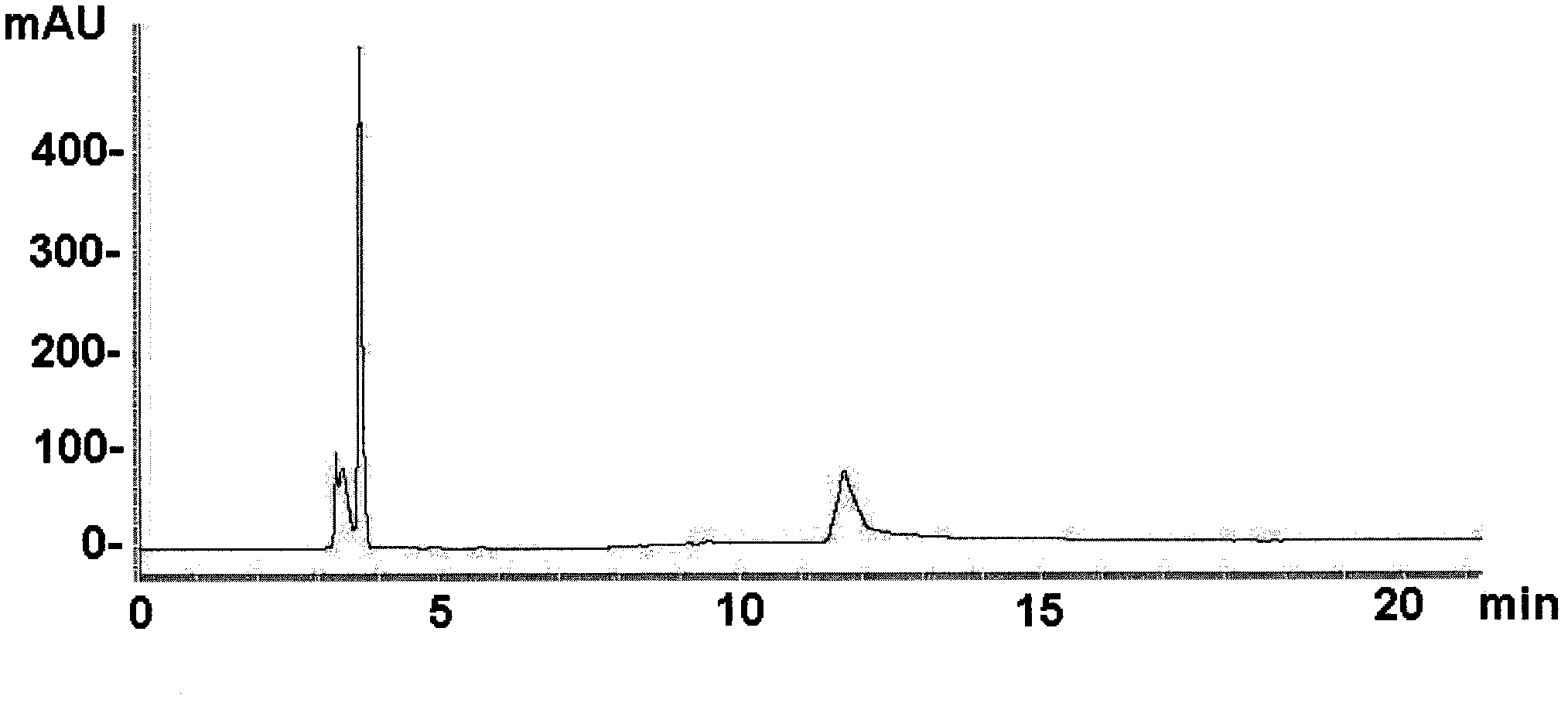
[0051] In this embodiment, the dye-affinity filler based on hydroxylated polymethacrylic resin is preferred to replace the affinity filler based on dextran. The former has the advantages of high hardness of the filler, low back pressure, and can withstand a larger process flow rate. Secondly, choose two ion-exchange chromatography methods, first use strong anion exchange chromatography, and then use weak cation exchange chromatography to replace reversed-phase chromatography and other chromatography methods that are complex in process and easily lead to protein denaturation. The schematic diagram of its method is as figure 1 shown.
[0052] Materials and Instruments
[0053] AF-Blue HC 650M filler (TOSOH company product), CM-sepharose FF filler (GE healthcare company product), Q-sepharose FF filler (GE healthcare company product). Pellicon 2 ultrafiltration membrane (molecular weight cut off 10...
Embodiment 3
[0067] Example 3 Purification Route B of Recombinant Human IFNβ-1a Purification
[0068] This embodiment includes two ion-exchange chromatography methods, preferably using weak cation-exchange chromatography first, followed by strong anion-exchange chromatography. The schematic diagram of its method is as figure 1 shown.
[0069] Materials and Instruments
[0070] Capto-Blue (product of GE healthcare company), CM-sepharose FF filler (product of GE healthcare company), Q-sepharose FF filler (product of GE healthcare company). Pellicon 2 ultrafiltration membrane (molecular weight cut off 10K), 0.5M 2 (Millipore company product), Pellicon ultrafiltration system (Millipore company product), Amicon ultrafiltration centrifuge tube (Millipore company product), AKTA Purifier chromatography system (GE healthcare company product).
[0071] experimental method
[0072] 1) Concentration of the supernatant
[0073] Clean the ultrafiltration system, concentrate the supernatant, and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Copy number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com