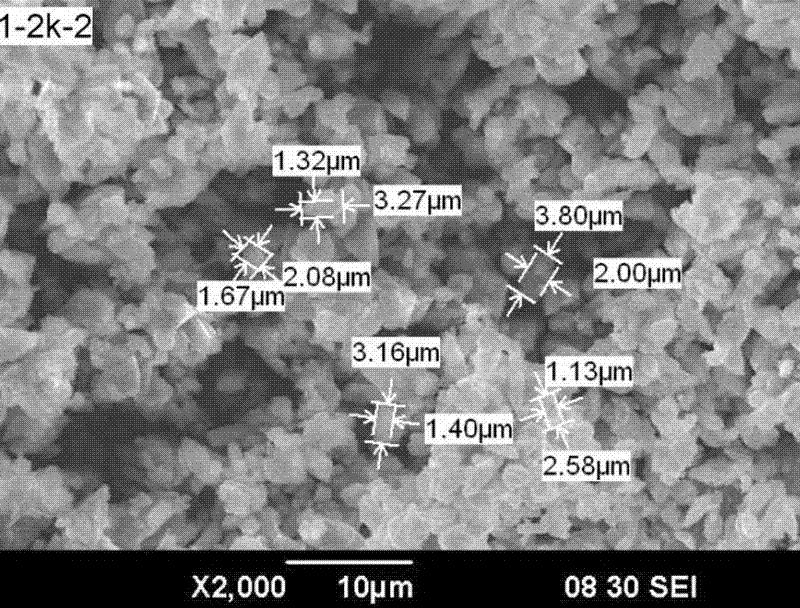
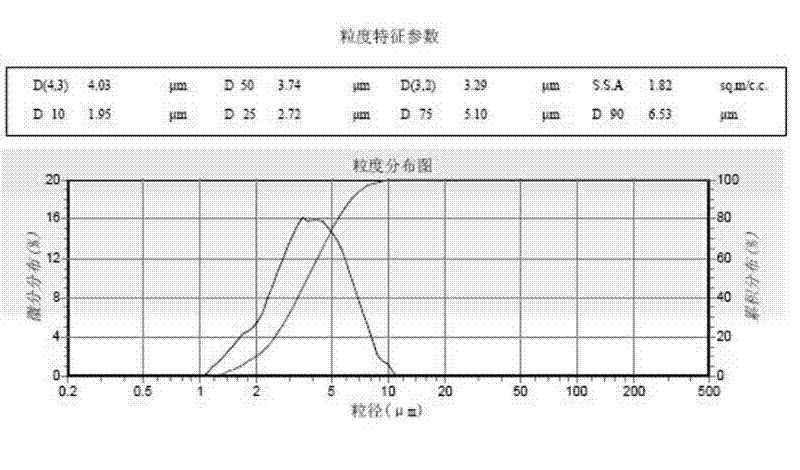
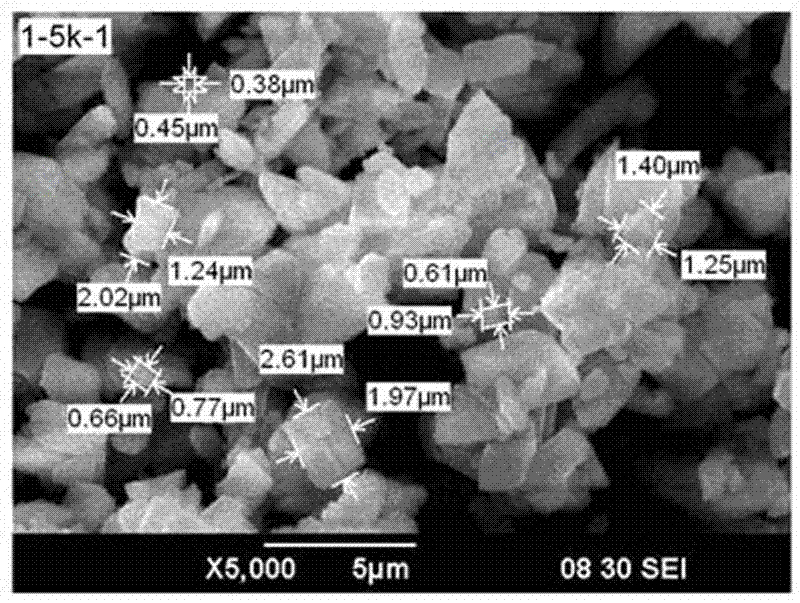
Method for preparing battery-level ferrous oxalate for production of lithium iron phosphate material
A technology of ferrous oxalate and lithium iron phosphate, which is applied in the direction of battery electrodes, carboxylate preparation, circuits, etc., can solve the problems of insufficient product quality, low LFP gram capacity, and inability to meet the raw material requirements for LFP battery production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 1500Kg of iron filings, add 2500L of industrial by-product 30% hydrochloric acid into a 5000L reactor, heat up to about 90 degrees, the reaction begins to intensify, and a large number of bubbles are generated. After maintaining the reaction liquid level for about 4 hours, the pH reached 2.5~3.5 and the reaction was almost terminated.
[0036] After plate and frame filtration, the iron salt solution is about 25 °Be', and the filtrate is pumped into a 6000L reactor and kept at 55-75 °C for use;
[0037] Prepare an oxalic acid solution with a concentration of 10-30%, filter it through a plate and frame, keep it warm and store it in a PP tank for use.
[0038] Turn on the iron salt stirring, and the stirring speed can be controlled by the frequency converter to about 50r / min, and the oxalic acid solution is quantitatively passed through the metering tank
[0039] (The amount of oxalic acid or oxalate added can be calculated by measuring the ferric ion content in the...
Embodiment 2
[0047] Weigh 1500Kg scrap iron scraps, add 2500L of industrial by-product 30% hydrochloric acid into a 5000L reactor, heat up to about 90 degrees, the reaction begins to intensify, and a large number of bubbles are generated. After maintaining the reaction liquid level for about 4 hours, the pH reached 2.5~3.5. The reaction was almost terminated.
[0048] After plate and frame filtration, the iron salt solution is about 25 °Be', and the filtrate is pumped into a 6000L reactor and kept at 55-75 °C for use;
[0049] Prepare an oxalic acid solution with a concentration of 10-30%, filter it through a plate and frame, keep it warm and store it in a PP tank for use.
[0050] Turn on the iron salt stirring, and control the stirring speed by the frequency converter to about 50r / min, and quantitatively pass the oxalic acid solution through the metering tank (the amount of oxalic acid or oxalate added can be calculated by measuring the ferrous ion content in the iron salt solution ) is s...
Embodiment 3
[0057] After weighing 1000Kg of waste iron oxide and 500KG of iron flakes or iron filings, add 2500L of industrial by-product 30% hydrochloric acid into a 5000L reactor, and raise the temperature to about 90 degrees, the reaction begins to intensify, and a large number of bubbles are generated. The reaction is accompanied by metathesis reactions and redox reactions and displacement reactions. Keeping the reaction liquid level for about 3 hours is faster than simply adding iron flakes, and the pH can reach 3.0~4.5, and the reaction is almost terminated at this time.
[0058] After the reaction solution with this ingredient is filtered through the plate frame, the iron salt solution can reach about 28 °Be', and the filtrate is pumped into a 6000L reactor and kept at 55-75 °C for use;
[0059] Prepare an oxalic acid solution with a concentration of 10-30%, filter it through a plate and frame, keep it warm and store it in a PP tank for use.
[0060] Turn on the iron salt stirring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com